You are browsing environment: FUNGIDB
CAZyme Information: Z517_11370-t43_1-p1
You are here: Home > Sequence: Z517_11370-t43_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Fonsecaea pedrosoi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Fonsecaea; Fonsecaea pedrosoi | |||||||||||
| CAZyme ID | Z517_11370-t43_1-p1 | |||||||||||
| CAZy Family | GT34 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 118 | 359 | 2.2e-49 | 0.5218340611353712 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396238 | FAD_binding_4 | 1.47e-12 | 146 | 257 | 26 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 223354 | GlcD | 1.01e-09 | 133 | 279 | 44 | 197 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 269987 | PTB_CCM2 | 0.005 | 12 | 105 | 97 | 191 | Cerebral cavernous malformation 2 FERM domain C-lobe. CCM2 (also called malcavernin; C7orf22/chromosome 7 open reading frame 22; OSM) along with CCM1 and CCM3 constitutes a set of proteins which when mutated are responsible for cerebral cavernous malformations, an autosomal dominant neurovascular disease characterized by cerebral hemorrhages and vascular malformations in the central nervous system. CCM2 plays many functional roles. CCM2 functions as a scaffold involved in small GTPase Rac-dependent p38 mitogen-activated protein kinase (MAPK) activation when the cell is under hyperosmotic stress. It associates with CCM1 in the signalling cascades that regulate vascular integrity and participates in HEG1 (the transmembrane receptor heart of glass 1) mediated endothelial cell junctions. CCM proteins also inhibit the activation of small GTPase RhoA and its downstream effector Rho kinase (ROCK) to limit vascular permeability. CCM2 mediates TrkA-dependent cell death via its N-terminal PTB domain in pediatric neuroblastic tumours. CCM2 possesses an N-terminal PTB domain and a C-terminal Karet domain. PTB domains have a common PH-like fold and are found in various eukaryotic signaling molecules. This domain was initially shown to binds peptides with a NPXY motif with differing requirements for phosphorylation of the tyrosine, although more recent studies have found that some types of PTB domains can bind to peptides lack tyrosine residues altogether. In contrast to SH2 domains, which recognize phosphotyrosine and adjacent carboxy-terminal residues, PTB-domain binding specificity is conferred by residues amino-terminal to the phosphotyrosine. PTB domains are classified into three groups: phosphotyrosine-dependent Shc-like, phosphotyrosine-dependent IRS-like, and phosphotyrosine-independent Dab-like PTB domains. This cd is part of the Dab-like subgroup. |
| 215242 | PLN02441 | 0.008 | 240 | 278 | 193 | 231 | cytokinin dehydrogenase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.37e-19 | 150 | 408 | 88 | 354 | |
| 1.84e-19 | 107 | 540 | 41 | 485 | |
| 7.93e-19 | 121 | 289 | 61 | 231 | |
| 7.93e-19 | 121 | 289 | 61 | 231 | |
| 1.01e-17 | 126 | 289 | 70 | 232 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.48e-26 | 121 | 543 | 45 | 457 | The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_B The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_C The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_D The crystal structure of EncM T139V mutant [Streptomyces maritimus] |
|
| 1.35e-25 | 121 | 543 | 45 | 457 | The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_B The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_C The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_D The crystal structure of EncM V135T mutant [Streptomyces maritimus] |
|
| 1.35e-25 | 121 | 543 | 45 | 457 | The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_B The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_C The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_D The crystal structure of EncM V135M mutant [Streptomyces maritimus] |
|
| 1.82e-25 | 121 | 543 | 45 | 457 | The crystal structure of EncM H138T mutant [Streptomyces maritimus],6FYE_B The crystal structure of EncM H138T mutant [Streptomyces maritimus] |
|
| 2.45e-25 | 121 | 543 | 45 | 457 | Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_B Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_C Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_D Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],6FOQ_A The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_B The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_C The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_D The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOW_A The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_B The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_C The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_D The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FP3_A The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_B The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_C The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_D The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FY8_A The crystal structure of EncM bromide soak [Streptomyces maritimus],6FY9_A The crystal structure of EncM complex with xenon under 15 bars Xe pressure [Streptomyces maritimus],6FYA_A The crystal structure of EncM under anaerobic conditions [Streptomyces maritimus],6FYA_B The crystal structure of EncM under anaerobic conditions [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.07e-79 | 75 | 550 | 29 | 518 | FAD-dependent monooxygenase tpcD OS=Cochliobolus heterostrophus (strain C5 / ATCC 48332 / race O) OX=701091 GN=tpcD PE=1 SV=1 |
|
| 6.56e-74 | 115 | 558 | 75 | 522 | FAD-dependent monooxygenase drtC OS=Aspergillus calidoustus OX=454130 GN=drtC PE=1 SV=1 |
|
| 7.83e-69 | 82 | 552 | 7 | 479 | FAD-dependent monooxygenase sdcF OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=sdcF PE=1 SV=1 |
|
| 2.08e-63 | 57 | 553 | 10 | 511 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti OX=5082 GN=prx3 PE=3 SV=1 |
|
| 2.08e-63 | 57 | 553 | 10 | 511 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti (strain FM164) OX=1365484 GN=prx3 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
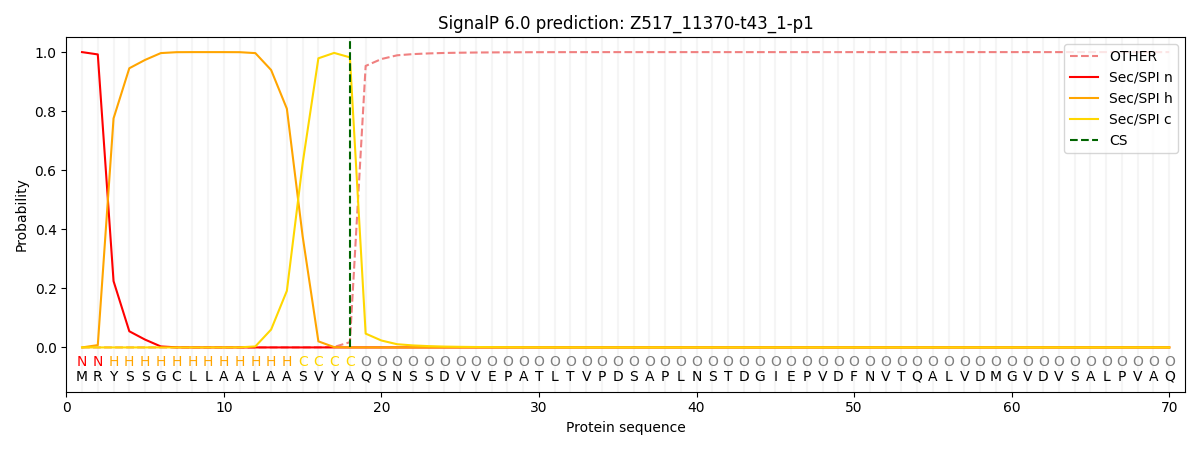
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000215 | 0.999743 | CS pos: 18-19. Pr: 0.9822 |
