You are browsing environment: FUNGIDB
CAZyme Information: Z517_07239-t43_1-p1
You are here: Home > Sequence: Z517_07239-t43_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
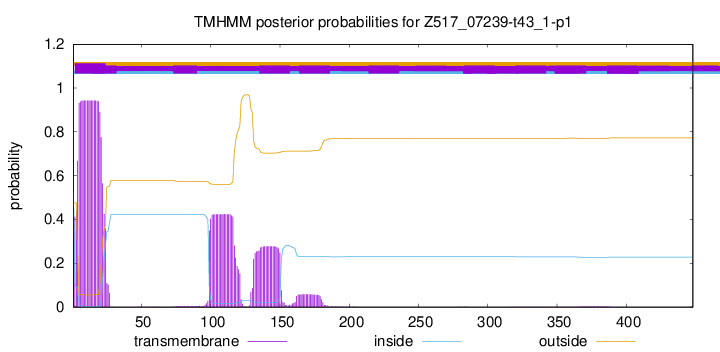
TMHMM annotations
Basic Information help
| Species | Fonsecaea pedrosoi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Fonsecaea; Fonsecaea pedrosoi | |||||||||||
| CAZyme ID | Z517_07239-t43_1-p1 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.142:10 | 2.4.1.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT33 | 31 | 442 | 3.1e-150 | 0.9929411764705882 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 340843 | GT33_ALG1-like | 0.0 | 32 | 444 | 5 | 411 | chitobiosyldiphosphodolichol beta-mannosyltransferase and similar proteins. This family is most closely related to the GT33 family of glycosyltransferases. The yeast gene ALG1 has been shown to function as a mannosyltransferase that catalyzes the formation of dolichol pyrophosphate (Dol-PP)-GlcNAc2Man from GDP-Man and Dol-PP-Glc-NAc2, and participates in the formation of the lipid-linked precursor oligosaccharide for N-glycosylation. In humans ALG1 has been associated with the congenital disorders of glycosylation (CDG) designated as subtype CDG-Ik. |
| 215155 | PLN02275 | 1.04e-139 | 33 | 410 | 7 | 371 | transferase, transferring glycosyl groups |
| 340831 | GT4_PimA-like | 1.26e-07 | 137 | 430 | 90 | 351 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| 340825 | GT4_WbuB-like | 2.29e-06 | 136 | 414 | 107 | 364 | Escherichia coli WbuB and similar proteins. This family is most closely related to the GT1 family of glycosyltransferases. WbuB in E. coli is involved in the biosynthesis of the O26 O-antigen. It has been proposed to function as an N-acetyl-L-fucosamine (L-FucNAc) transferase. |
| 340816 | Glycosyltransferase_GTB-type | 3.37e-04 | 261 | 393 | 114 | 232 | glycosyltransferase family 1 and related proteins with GTB topology. Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. The structures of the formed glycoconjugates are extremely diverse, reflecting a wide range of biological functions. The members of this family share a common GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.88e-182 | 2 | 446 | 5 | 461 | |
| 9.57e-178 | 10 | 446 | 13 | 458 | |
| 1.97e-177 | 3 | 446 | 19 | 490 | |
| 7.03e-176 | 9 | 446 | 12 | 460 | |
| 5.52e-175 | 2 | 446 | 5 | 461 |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.81e-134 | 27 | 448 | 34 | 447 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_01551 PE=3 SV=1 |
|
| 7.80e-95 | 35 | 446 | 44 | 440 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Yarrowia lipolytica (strain CLIB 122 / E 150) OX=284591 GN=ALG1 PE=3 SV=1 |
|
| 7.06e-90 | 35 | 443 | 9 | 454 | UDP-glycosyltransferase TURAN OS=Arabidopsis thaliana OX=3702 GN=TUN PE=2 SV=1 |
|
| 2.10e-87 | 25 | 439 | 20 | 415 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=alg1 PE=3 SV=2 |
|
| 2.08e-86 | 27 | 444 | 48 | 452 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=ALG1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
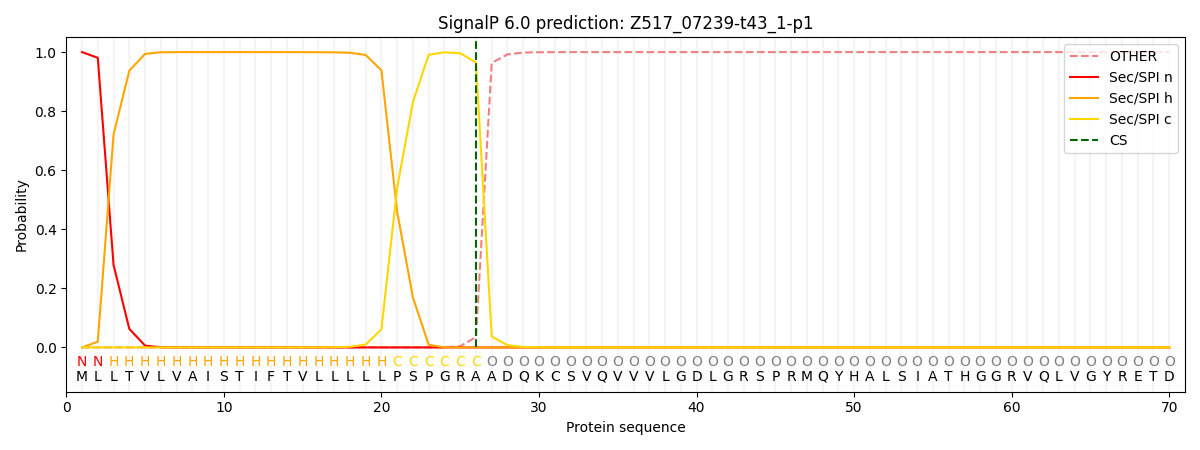
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000639 | 0.999336 | CS pos: 26-27. Pr: 0.9649 |