You are browsing environment: FUNGIDB
CAZyme Information: XP_001912281.1
You are here: Home > Sequence: XP_001912281.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Podospora anserina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Podosporaceae; Podospora; Podospora anserina | |||||||||||
| CAZyme ID | XP_001912281.1 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | SGNH_hydro domain-containing protein [Source:UniProtKB/TrEMBL;Acc:B2A9L3] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE3 | 27 | 222 | 6.2e-45 | 0.9948453608247423 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 238871 | XynB_like | 2.55e-38 | 27 | 222 | 1 | 157 | SGNH_hydrolase subfamily, similar to Ruminococcus flavefaciens XynB. Most likely a secreted hydrolase with xylanase activity. SGNH hydrolases are a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| 238141 | SGNH_hydrolase | 5.65e-13 | 32 | 221 | 4 | 187 | SGNH_hydrolase, or GDSL_hydrolase, is a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxlic acid. |
| 404371 | Lipase_GDSL_2 | 1.34e-11 | 32 | 210 | 2 | 173 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| 238866 | sialate_O-acetylesterase_like2 | 1.00e-09 | 32 | 222 | 5 | 168 | sialate_O-acetylesterase_like subfamily of the SGNH-hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| 238879 | NnaC_like | 3.11e-07 | 108 | 215 | 53 | 167 | NnaC (CMP-NeuNAc synthetase) _like subfamily of SGNH_hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles two of the three components of typical Ser-His-Asp(Glu) triad from other serine hydrolases. E. coli NnaC appears to be involved in polysaccharide synthesis. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.52e-173 | 1 | 234 | 1 | 234 | |
| 2.52e-173 | 1 | 234 | 1 | 234 | |
| 3.98e-171 | 1 | 234 | 1 | 237 | |
| 3.95e-58 | 27 | 230 | 25 | 227 | |
| 1.28e-46 | 25 | 223 | 162 | 362 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.00e-29 | 27 | 230 | 2 | 207 | Crystal structure of a carbohydrate esterase family 3 from Talaromyces cellulolyticus [Talaromyces cellulolyticus] |
|
| 5.55e-29 | 27 | 230 | 2 | 207 | Crystal structure of acetyl esterase mutant S10A with acetate ion [Talaromyces cellulolyticus] |
|
| 1.60e-16 | 23 | 231 | 2 | 204 | Chain A, LIPOLYTIC ENZYME [Acetivibrio thermocellus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.67e-09 | 18 | 214 | 33 | 252 | Multidomain esterase OS=Ruminococcus flavefaciens OX=1265 GN=cesA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
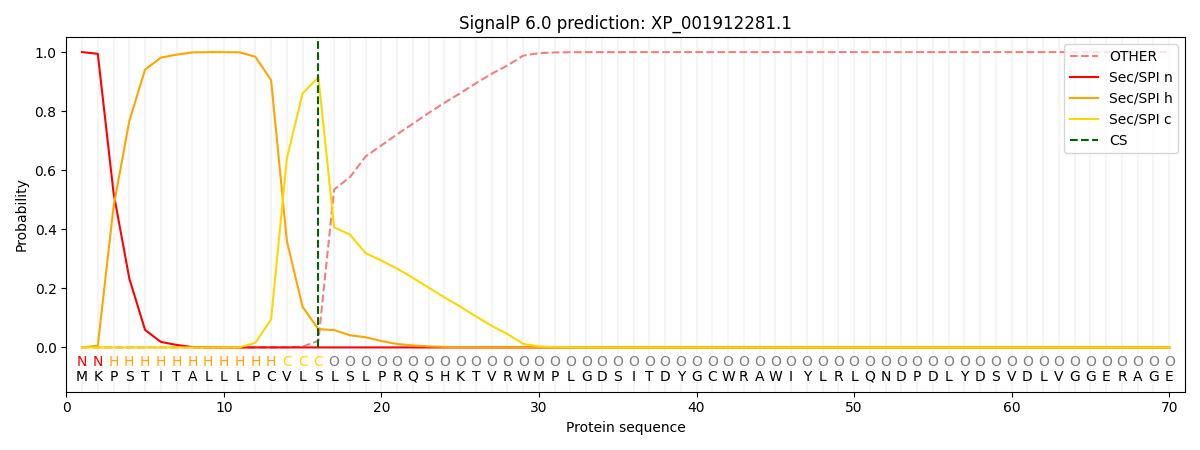
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000488 | 0.999483 | CS pos: 16-17. Pr: 0.9152 |
