You are browsing environment: FUNGIDB
CAZyme Information: VBB72927.1
You are here: Home > Sequence: VBB72927.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Podospora comata | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Podosporaceae; Podospora; Podospora comata | |||||||||||
| CAZyme ID | VBB72927.1 | |||||||||||
| CAZy Family | AA4 | |||||||||||
| CAZyme Description | Putative Glycoside Hydrolase Family 35 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.23:18 | 2.4.1.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH35 | 109 | 440 | 8.7e-91 | 0.9804560260586319 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396048 | Glyco_hydro_35 | 4.12e-89 | 112 | 440 | 6 | 313 | Glycosyl hydrolases family 35. |
| 402180 | BetaGal_dom2 | 7.24e-43 | 471 | 631 | 14 | 180 | Beta-galactosidase, domain 2. This is the second domain of the five-domain beta-galactosidase enzyme that altogether catalyzes the hydrolysis of beta(1-3) and beta(1-4) galactosyl bonds in oligosaccharides as well as the inverse reaction of enzymatic condensation and trans-glycosylation. This domain is made up of 16 antiparallel beta-strands and an alpha-helix at its C-terminus. The fold of this domain appears to be unique. In addition, the last seven strands of the domain form a subdomain with an immunoglobulin-like (I-type Ig) fold in which the first strand is divided between the two beta-sheets. In penicillin spp this strand is interrupted by a 12-residue insertion which forms an additional edge-strand to the second beta-sheet of the sub-domain. The remainder of the second domain forms a series of beta-hairpins at its N-terminus, four strands of which are contiguous with part of the Ig-like sub-domain, forming in total a seven-stranded antiparallel beta-sheet. This domain is associated with family Glyco_hydro_35, pfam01301, which is N-terminal to it, but itself has no metazoan members. |
| 166698 | PLN03059 | 7.07e-34 | 100 | 439 | 29 | 336 | beta-galactosidase; Provisional |
| 198097 | BetaGal_dom2 | 1.97e-26 | 459 | 632 | 6 | 182 | Beta-galactosidase, domain 2. This is the second domain of the five-domain beta-galactosidase enzyme that altogether catalyses the hydrolysis of beta(1-3) and beta(1-4) galactosyl bonds in oligosaccharides as well as the inverse reaction of enzymatic condensation and trans-glycosylation. This domain is made up of 16 antiparallel beta-strands and an alpha-helix at its C terminus. The fold of this domain appears to be unique. In addition, the last seven strands of the domain form a subdomain with an immunoglobulin-like (I-type Ig) fold in which the first strand is divided between the two beta-sheets. In penicillin spp this strand is interrupted by a 12-residue insertion which forms an additional edge-strand to the second beta-sheet of the sub-domain. The remainder of the second domain forms a series of beta-hairpins at its N terminus, four strands of which are contiguous with part of the Ig-like sub-domain, forming in total a seven-stranded antiparallel beta-sheet. This domain is associated with family Glyco_hydro_35, which is N-terminal to it, but itself has no metazoan members. |
| 404274 | BetaGal_dom4_5 | 1.79e-25 | 926 | 1039 | 1 | 107 | Beta-galactosidase jelly roll domain. This domain is found in beta galactosidase enzymes. It has a jelly roll fold. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 1084 | 1 | 1084 | |
| 0.0 | 1 | 1084 | 1 | 1082 | |
| 0.0 | 1 | 1084 | 1 | 1082 | |
| 3.09e-299 | 101 | 1084 | 44 | 992 | |
| 1.13e-291 | 101 | 1084 | 45 | 1015 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.15e-289 | 101 | 1084 | 26 | 1003 | Chain A, Beta-galactosidase [Trichoderma reesei],3OGR_A Chain A, Beta-galactosidase [Trichoderma reesei],3OGS_A Chain A, Beta-galactosidase [Trichoderma reesei],3OGV_A Chain A, Beta-galactosidase [Trichoderma reesei] |
|
| 1.03e-275 | 101 | 1084 | 46 | 1007 | Structure Of Beta-galactosidase From Aspergillus Niger [Aspergillus niger CBS 513.88] |
|
| 2.90e-275 | 101 | 1084 | 46 | 1007 | STRUCTURE OF E298Q-BETA-GALACTOSIDASE FROM ASPERGILLUS NIGER IN COMPLEX WITH 3-b-Galactopyranosyl glucose [Aspergillus niger CBS 513.88],5IHR_A Structure Of E298q-beta-galactosidase From Aspergillus Niger In Complex With Allolactose [Aspergillus niger CBS 513.88],5JUV_A STRUCTURE OF E298Q-BETA-GALACTOSIDASE FROM ASPERGILLUS NIGER IN COMPLEX WITH 6-b-Galactopyranosyl galactose [Aspergillus niger CBS 513.88],5MGC_A STRUCTURE OF E298Q-BETA-GALACTOSIDASE FROM ASPERGILLUS NIGER IN COMPLEX WITH 4-Galactosyl-lactose [Aspergillus niger CBS 513.88],5MGD_A STRUCTURE OF E298Q-BETA-GALACTOSIDASE FROM ASPERGILLUS NIGER IN COMPLEX WITH 6-Galactosyl-lactose [Aspergillus niger CBS 513.88] |
|
| 1.19e-271 | 101 | 1084 | 6 | 971 | Native structure of beta-galactosidase from Penicillium sp. [Penicillium sp.],1XC6_A Native Structure Of Beta-Galactosidase from Penicillium sp. in complex with Galactose [Penicillium sp.] |
|
| 3.24e-269 | 101 | 1084 | 46 | 1005 | Crystal structure of beta-galactosidase from Aspergillus oryzae in complex with galactose [Aspergillus oryzae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.35e-275 | 101 | 1084 | 46 | 1007 | Beta-galactosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=lacA PE=1 SV=1 |
|
| 7.81e-273 | 101 | 1084 | 46 | 1007 | Probable beta-galactosidase A OS=Aspergillus phoenicis OX=5063 GN=lacA PE=2 SV=1 |
|
| 2.25e-270 | 101 | 1084 | 46 | 1011 | Beta-galactosidase A OS=Penicillium sp. OX=5081 GN=lacA PE=1 SV=1 |
|
| 2.71e-270 | 101 | 1084 | 46 | 1006 | Probable beta-galactosidase A OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=lacA PE=3 SV=2 |
|
| 7.65e-270 | 101 | 1084 | 46 | 1006 | Probable beta-galactosidase A OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=lacA PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
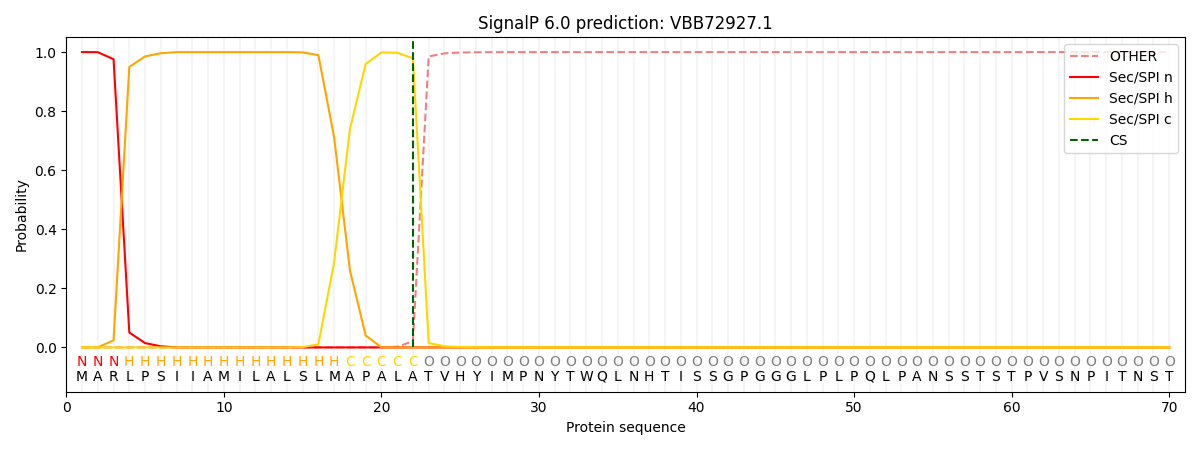
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000200 | 0.999785 | CS pos: 22-23. Pr: 0.9789 |

