You are browsing environment: FUNGIDB
CAZyme Information: TSTA_053270-t26_1-p1
You are here: Home > Sequence: TSTA_053270-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Talaromyces stipitatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Trichocomaceae; Talaromyces; Talaromyces stipitatus | |||||||||||
| CAZyme ID | TSTA_053270-t26_1-p1 | |||||||||||
| CAZy Family | GH172 | |||||||||||
| CAZyme Description | alpha-galactosidase, putative | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.49:3 | 3.2.1.22:2 | 3.2.1.49:2 | 3.2.1.22:2 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH27 | 135 | 404 | 1.1e-53 | 0.9868995633187773 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 269893 | GH27 | 2.31e-102 | 41 | 333 | 1 | 271 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| 166449 | PLN02808 | 2.27e-76 | 27 | 424 | 18 | 381 | alpha-galactosidase |
| 374582 | Melibiase_2 | 3.92e-75 | 40 | 333 | 1 | 284 | Alpha galactosidase A. |
| 178295 | PLN02692 | 7.42e-66 | 36 | 405 | 51 | 386 | alpha-galactosidase |
| 177874 | PLN02229 | 5.21e-63 | 34 | 405 | 56 | 395 | alpha-galactosidase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.56e-245 | 19 | 554 | 10 | 531 | |
| 8.42e-208 | 13 | 554 | 17 | 535 | |
| 2.76e-204 | 5 | 554 | 2 | 527 | |
| 5.89e-196 | 43 | 549 | 1 | 487 | |
| 3.22e-189 | 15 | 554 | 6 | 520 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.16e-63 | 36 | 424 | 4 | 357 | Chain A, alpha-galactosidase [Oryza sativa] |
|
| 1.20e-53 | 36 | 382 | 4 | 342 | Chain A, Alpha-galactosidase A [Homo sapiens],3LX9_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXA_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXA_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXB_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXB_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXC_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXC_B Chain B, Alpha-galactosidase A [Homo sapiens] |
|
| 2.02e-52 | 36 | 382 | 4 | 342 | Structure of human alpha-galactosidase [Homo sapiens],1R46_B Structure of human alpha-galactosidase [Homo sapiens],1R47_A Structure of human alpha-galactosidase [Homo sapiens],1R47_B Structure of human alpha-galactosidase [Homo sapiens],3GXN_A Crystal structure of apo alpha-galactosidase A at pH 4.5 [Homo sapiens],3GXN_B Crystal structure of apo alpha-galactosidase A at pH 4.5 [Homo sapiens],3GXP_A Crystal structure of acid-alpha-galactosidase A complexed with galactose at pH 4.5 [Homo sapiens],3GXP_B Crystal structure of acid-alpha-galactosidase A complexed with galactose at pH 4.5 [Homo sapiens],3GXT_A Crystal structure of alpha-galactosidase A at pH 4.5 complexed with 1-deoxygalactonijirimycin [Homo sapiens],3GXT_B Crystal structure of alpha-galactosidase A at pH 4.5 complexed with 1-deoxygalactonijirimycin [Homo sapiens],3HG2_A Human alpha-galactosidase catalytic mechanism 1. Empty active site [Homo sapiens],3HG2_B Human alpha-galactosidase catalytic mechanism 1. Empty active site [Homo sapiens],3HG4_A Human alpha-galactosidase catalytic mechanism 3. Covalent intermediate [Homo sapiens],3HG4_B Human alpha-galactosidase catalytic mechanism 3. Covalent intermediate [Homo sapiens],3HG5_A Human alpha-galactosidase catalytic mechanism 4. Product bound [Homo sapiens],3HG5_B Human alpha-galactosidase catalytic mechanism 4. Product bound [Homo sapiens],3S5Y_A Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Y_B Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Z_A Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Z_B Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],4NXS_A Crystal structure of human alpha-galactosidase A in complex with 1-deoxygalactonojirimycin-pFPhT [Homo sapiens],4NXS_B Crystal structure of human alpha-galactosidase A in complex with 1-deoxygalactonojirimycin-pFPhT [Homo sapiens],6IBK_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfamidate ME763 [Homo sapiens],6IBK_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfamidate ME763 [Homo sapiens],6IBM_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfate ME776 [Homo sapiens],6IBM_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfate ME776 [Homo sapiens],6IBR_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol epoxide LWA481 [Homo sapiens],6IBR_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol epoxide LWA481 [Homo sapiens],6IBT_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol aziridine ME737 [Homo sapiens],6IBT_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol aziridine ME737 [Homo sapiens] |
|
| 2.94e-52 | 37 | 366 | 96 | 419 | Crystal structure of a putative alpha-galactosidase/melibiase (BF4189) from Bacteroides fragilis NCTC 9343 at 2.00 A resolution [Bacteroides fragilis NCTC 9343],4OGZ_B Crystal structure of a putative alpha-galactosidase/melibiase (BF4189) from Bacteroides fragilis NCTC 9343 at 2.00 A resolution [Bacteroides fragilis NCTC 9343] |
|
| 8.90e-52 | 32 | 405 | 1 | 361 | The Structure of alpha-N-Acetylgalactosaminidase [Gallus gallus],1KTC_A The Structure of alpha-N-Acetylgalactosaminidase [Gallus gallus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.50e-208 | 13 | 554 | 17 | 535 | Alpha-galactosidase A OS=Aspergillus niger OX=5061 GN=aglA PE=1 SV=1 |
|
| 4.90e-205 | 5 | 554 | 2 | 527 | Probable alpha-galactosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=aglA PE=3 SV=1 |
|
| 5.73e-190 | 15 | 554 | 6 | 520 | Probable alpha-galactosidase A OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=aglA PE=3 SV=1 |
|
| 1.63e-189 | 24 | 554 | 15 | 520 | Probable alpha-galactosidase A OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=aglA PE=3 SV=1 |
|
| 7.47e-186 | 14 | 554 | 8 | 523 | Probable alpha-galactosidase A OS=Aspergillus flavus (strain ATCC 200026 / FGSC A1120 / IAM 13836 / NRRL 3357 / JCM 12722 / SRRC 167) OX=332952 GN=aglA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
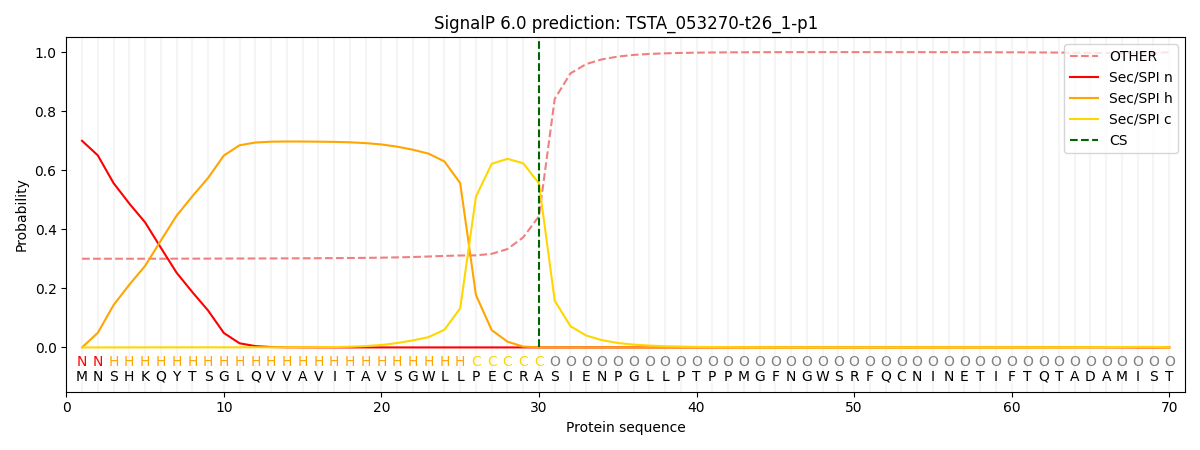
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.325441 | 0.674551 | CS pos: 30-31. Pr: 0.5553 |
