You are browsing environment: FUNGIDB
CAZyme Information: SDRG_14915-t26_1-p1
You are here: Home > Sequence: SDRG_14915-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Saprolegnia diclina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Saprolegniaceae; Saprolegnia; Saprolegnia diclina | |||||||||||
| CAZyme ID | SDRG_14915-t26_1-p1 | |||||||||||
| CAZy Family | GT2|GT2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 46 | 293 | 9.6e-28 | 0.9058823529411765 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173825 | ascorbate_peroxidase | 3.66e-28 | 51 | 297 | 33 | 251 | Ascorbate peroxidases and cytochrome C peroxidases. Ascorbate peroxidases are a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Along with related catalase-peroxidases, ascorbate peroxidases belong to class I of the plant superfamily. Ascorbate peroxidases are found in the chloroplasts and/or cytosol of algae and plants, where they have been shown to control the concentration of lethal hydrogen peroxide molecules. The yeast cytochrome c peroxidase is a divergent member of the family; it forms a complex with cytochrome c to catalyze the reduction of hydrogen peroxide to water. |
| 178218 | PLN02608 | 2.50e-21 | 51 | 295 | 34 | 243 | L-ascorbate peroxidase |
| 166005 | PLN02364 | 3.49e-15 | 51 | 295 | 36 | 246 | L-ascorbate peroxidase 1 |
| 173827 | secretory_peroxidase | 1.10e-14 | 48 | 295 | 33 | 282 | Horseradish peroxidase and related secretory plant peroxidases. Secretory peroxidases belong to class III of the plant heme-dependent peroxidase superfamily. All members of the superfamily share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Class III peroxidases are found in the extracellular space or in the vacuole in plants where they have been implicated in hydrogen peroxide detoxification, auxin catabolism and lignin biosynthesis, and stress response. Class III peroxidases contain four conserved disulphide bridges and two conserved calcium binding sites. |
| 178467 | PLN02879 | 4.96e-14 | 51 | 295 | 37 | 246 | L-ascorbate peroxidase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.30e-17 | 23 | 295 | 14 | 246 | |
| 2.29e-17 | 23 | 295 | 13 | 244 | |
| 6.02e-17 | 23 | 295 | 12 | 243 | |
| 1.12e-16 | 23 | 295 | 12 | 243 | |
| 2.14e-16 | 23 | 303 | 12 | 252 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.00e-16 | 22 | 295 | 12 | 245 | CRYSTAL STRUCTURE OF RECOMBINANT ASCORBATE PEROXIDASE [Pisum sativum],1APX_B CRYSTAL STRUCTURE OF RECOMBINANT ASCORBATE PEROXIDASE [Pisum sativum],1APX_C CRYSTAL STRUCTURE OF RECOMBINANT ASCORBATE PEROXIDASE [Pisum sativum],1APX_D CRYSTAL STRUCTURE OF RECOMBINANT ASCORBATE PEROXIDASE [Pisum sativum] |
|
| 4.82e-16 | 102 | 299 | 85 | 262 | Structure of Leishmania major peroxidase D211N mutant [Leishmania major],5AMM_B Structure of Leishmania major peroxidase D211N mutant [Leishmania major] |
|
| 6.43e-16 | 102 | 299 | 85 | 262 | Crystal Structure of the Leishmania Major Peroxidase-Cytochrome C Complex [Leishmania major] |
|
| 6.60e-16 | 102 | 299 | 85 | 262 | Structure of Leishmania major peroxidase D211R mutant (high res) [Leishmania major],5ALA_A Structure of Leishmania major peroxidase D211R mutant (low res) [Leishmania major],5ALA_B Structure of Leishmania major peroxidase D211R mutant (low res) [Leishmania major] |
|
| 6.69e-16 | 102 | 299 | 86 | 263 | The Crystal Structure of Leishmania major Peroxidase mutant C197T [Leishmania major strain Friedlin],3RIW_B The Crystal Structure of Leishmania major Peroxidase mutant C197T [Leishmania major strain Friedlin] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.02e-16 | 18 | 295 | 9 | 246 | L-ascorbate peroxidase 1, cytosolic OS=Oryza sativa subsp. indica OX=39946 GN=APX1 PE=2 SV=1 |
|
| 3.42e-16 | 51 | 295 | 34 | 243 | L-ascorbate peroxidase 3 OS=Arabidopsis thaliana OX=3702 GN=APX3 PE=1 SV=1 |
|
| 5.24e-16 | 22 | 295 | 13 | 246 | L-ascorbate peroxidase, cytosolic OS=Pisum sativum OX=3888 GN=APX1 PE=1 SV=2 |
|
| 6.61e-15 | 18 | 295 | 9 | 246 | L-ascorbate peroxidase 1, cytosolic OS=Oryza sativa subsp. japonica OX=39947 GN=APX1 PE=1 SV=1 |
|
| 1.50e-14 | 51 | 296 | 116 | 345 | Cytochrome c peroxidase, mitochondrial OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=ccp1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
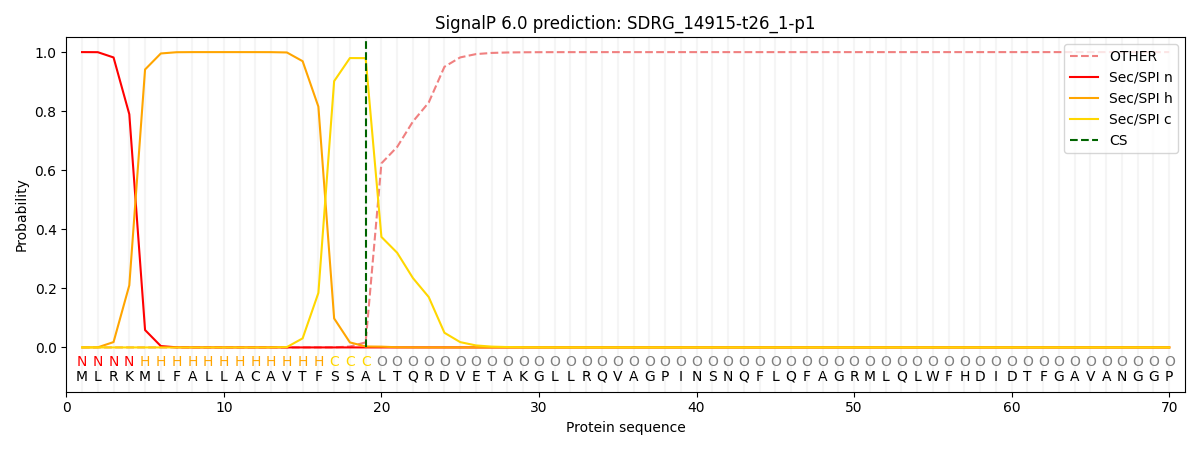
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000219 | 0.999741 | CS pos: 19-20. Pr: 0.9796 |
