You are browsing environment: FUNGIDB
CAZyme Information: SDRG_14569-t26_1-p1
You are here: Home > Sequence: SDRG_14569-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
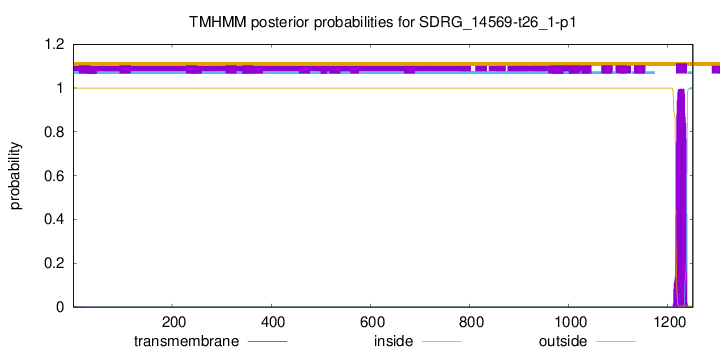
TMHMM annotations
Basic Information help
| Species | Saprolegnia diclina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Saprolegniaceae; Saprolegnia; Saprolegnia diclina | |||||||||||
| CAZyme ID | SDRG_14569-t26_1-p1 | |||||||||||
| CAZy Family | GT22 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.39:2 | 3.2.1.39:2 | 3.2.1.-:2 | 3.2.1.39:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH16 | 26 | 374 | 2.5e-101 | 0.9968354430379747 |
| GH16 | 455 | 756 | 4.1e-76 | 0.9968354430379747 |
| GH16 | 794 | 1001 | 1.9e-37 | 0.6835443037974683 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 185694 | GH16_CCF | 6.47e-136 | 22 | 374 | 1 | 329 | Coelomic cytolytic factor, member of glycosyl hydrolase family 16. Subgroup of glucanases of unknown function that are related to beta-GRP (beta-1,3-glucan recognition protein), but contain active site residues. Beta-GRPs are one group of pattern recognition receptors (PRRs), also referred to as biosensor proteins, that complexes with pathogen-associated beta-1,3-glucans and then transduces signals necessary for activation of an appropriate innate immune response. Beta-GRPs are present in insects and lack all catalytic residues. This subgroup contains related proteins that still contain the active site and are widely distributed in eukaryotes. Their structures adopt a jelly roll fold with a deep active site channel harboring the catalytic residues, like those of other glycosyl hydrolase family 16 members. |
| 185694 | GH16_CCF | 3.91e-80 | 451 | 757 | 1 | 330 | Coelomic cytolytic factor, member of glycosyl hydrolase family 16. Subgroup of glucanases of unknown function that are related to beta-GRP (beta-1,3-glucan recognition protein), but contain active site residues. Beta-GRPs are one group of pattern recognition receptors (PRRs), also referred to as biosensor proteins, that complexes with pathogen-associated beta-1,3-glucans and then transduces signals necessary for activation of an appropriate innate immune response. Beta-GRPs are present in insects and lack all catalytic residues. This subgroup contains related proteins that still contain the active site and are widely distributed in eukaryotes. Their structures adopt a jelly roll fold with a deep active site channel harboring the catalytic residues, like those of other glycosyl hydrolase family 16 members. |
| 185688 | GH16_beta_GRP | 1.53e-69 | 22 | 375 | 1 | 321 | beta-1,3-glucan recognition protein, member of glycosyl hydrolase family 16. Beta-GRP (beta-1,3-glucan recognition protein) is one of several pattern recognition receptors (PRRs), also referred to as biosensor proteins, that complexes with pathogen-associated beta-1,3-glucans and then transduces signals necessary for activation of an appropriate innate immune response. They are present in insects and lack all catalytic residues. This subgroup also contains related proteins of unknown function that still contain the active site. Their structures adopt a jelly roll fold with a deep active site channel harboring the catalytic residues, like those of other glycosyl hydrolase family 16 members. |
| 185688 | GH16_beta_GRP | 1.55e-55 | 451 | 757 | 1 | 321 | beta-1,3-glucan recognition protein, member of glycosyl hydrolase family 16. Beta-GRP (beta-1,3-glucan recognition protein) is one of several pattern recognition receptors (PRRs), also referred to as biosensor proteins, that complexes with pathogen-associated beta-1,3-glucans and then transduces signals necessary for activation of an appropriate innate immune response. They are present in insects and lack all catalytic residues. This subgroup also contains related proteins of unknown function that still contain the active site. Their structures adopt a jelly roll fold with a deep active site channel harboring the catalytic residues, like those of other glycosyl hydrolase family 16 members. |
| 185693 | GH16_laminarinase_like | 8.29e-53 | 24 | 310 | 1 | 212 | Laminarinase, member of the glycosyl hydrolase family 16. Laminarinase, also known as glucan endo-1,3-beta-D-glucosidase, is a glycosyl hydrolase family 16 member that hydrolyzes 1,3-beta-D-glucosidic linkages in 1,3-beta-D-glucans such as laminarins, curdlans, paramylons, and pachymans, with very limited action on mixed-link (1,3-1,4-)-beta-D-glucans. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.67e-124 | 22 | 757 | 45 | 728 | |
| 1.32e-86 | 3 | 385 | 10 | 364 | |
| 1.98e-86 | 22 | 376 | 46 | 384 | |
| 7.63e-83 | 15 | 374 | 34 | 369 | |
| 8.11e-83 | 22 | 376 | 40 | 374 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.33e-20 | 22 | 309 | 9 | 218 | The crystal structure of an endo-beta-1,3-glucanase from alkaliphilic Nocardiopsis sp.strain F96 [Nocardiopsis sp. F96] |
|
| 2.46e-19 | 22 | 309 | 5 | 214 | endo-1,3-beta-glucanase from Cellulosimicrobium cellulans [Cellulosimicrobium cellulans] |
|
| 4.13e-19 | 22 | 309 | 20 | 238 | Structure of the catalytic domain of an endo-1,3-beta-glucanase (laminarinase) from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],4DFS_B Structure of the catalytic domain of an endo-1,3-beta-glucanase (laminarinase) from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1] |
|
| 4.54e-19 | 22 | 309 | 12 | 230 | Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZX_B Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZY_A Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZY_B Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZY_C Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZY_D Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3AZZ_A Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with gluconolactone [Thermotoga maritima MSB8],3AZZ_B Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with gluconolactone [Thermotoga maritima MSB8],3AZZ_C Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with gluconolactone [Thermotoga maritima MSB8],3AZZ_D Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with gluconolactone [Thermotoga maritima MSB8],3B00_A Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with cetyltrimethylammonium bromide [Thermotoga maritima MSB8],3B00_B Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with cetyltrimethylammonium bromide [Thermotoga maritima MSB8],3B00_C Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with cetyltrimethylammonium bromide [Thermotoga maritima MSB8],3B00_D Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with cetyltrimethylammonium bromide [Thermotoga maritima MSB8],3B01_A Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3B01_B Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3B01_C Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8],3B01_D Crystal structure of the laminarinase catalytic domain from Thermotoga maritima MSB8 [Thermotoga maritima MSB8] |
|
| 3.22e-15 | 120 | 323 | 129 | 291 | Chain AAA, Glycoside hydrolase family 16 protein [Akkermansia muciniphila],6T2N_BBB Chain BBB, Glycoside hydrolase family 16 protein [Akkermansia muciniphila] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.32e-80 | 22 | 374 | 32 | 359 | Beta-1,3-glucan-binding protein OS=Penaeus monodon OX=6687 PE=2 SV=1 |
|
| 6.56e-33 | 452 | 755 | 169 | 478 | Beta-1,3-glucan-binding protein OS=Tenebrio molitor OX=7067 GN=GRP PE=1 SV=1 |
|
| 2.62e-32 | 450 | 757 | 184 | 494 | Beta-1,3-glucan-binding protein OS=Bombyx mori OX=7091 PE=1 SV=1 |
|
| 2.57e-31 | 450 | 757 | 178 | 489 | Beta-1,3-glucan-binding protein 1 OS=Galleria mellonella OX=7137 GN=bgrp1 PE=1 SV=1 |
|
| 9.19e-30 | 450 | 760 | 174 | 494 | Gram-negative bacteria-binding protein 1 OS=Drosophila melanogaster OX=7227 GN=GNBP1 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
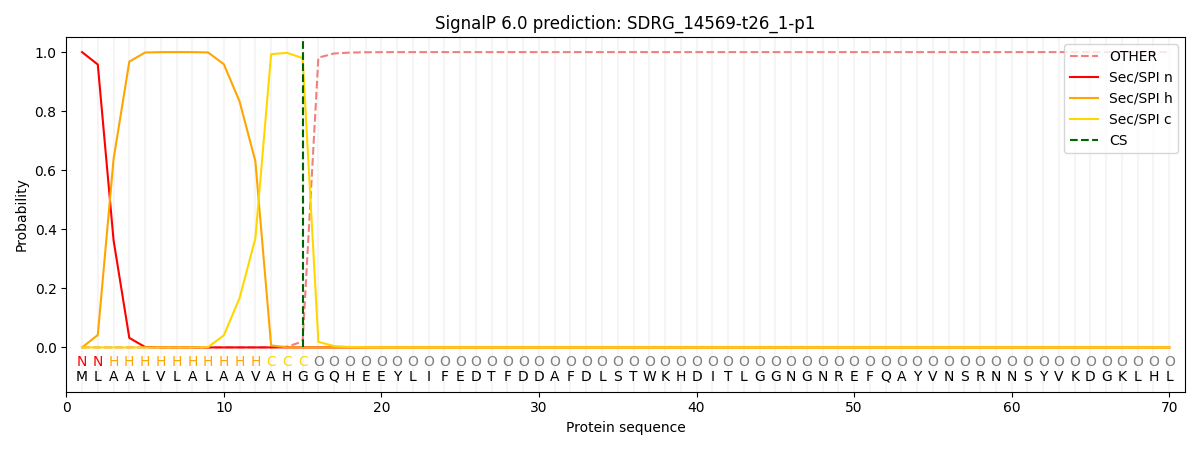
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000273 | 0.999720 | CS pos: 15-16. Pr: 0.9798 |