You are browsing environment: FUNGIDB
CAZyme Information: RQM13448.1
You are here: Home > Sequence: RQM13448.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Peronospora effusa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Peronospora; Peronospora effusa | |||||||||||
| CAZyme ID | RQM13448.1 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY [Source:UniProtKB/TrEMBL;Acc:A0A425C913] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT41 | 417 | 700 | 1e-93 | 0.42836879432624114 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 404688 | Glyco_transf_41 | 1.19e-170 | 468 | 899 | 1 | 543 | Glycosyl transferase family 41. This family of glycosyltransferases includes O-linked beta-N-acetylglucosamine (O-GlcNAc) transferase, an enzyme which catalyzes the addition of O-GlcNAc to serine and threonine residues. In addition to its function as an O-GlcNAc transferase, human OGT also appears to proteolytically cleave the epigenetic cell-cycle regulator HCF-1. |
| 226428 | Spy | 3.52e-109 | 370 | 911 | 84 | 619 | Predicted O-linked N-acetylglucosamine transferase, SPINDLY family [Posttranslational modification, protein turnover, chaperones]. |
| 274350 | PEP_TPR_lipo | 4.73e-34 | 23 | 452 | 271 | 734 | putative PEP-CTERM system TPR-repeat lipoprotein. This protein family occurs in strictly within a subset of Gram-negative bacterial species with the proposed PEP-CTERM/exosortase system, analogous to the LPXTG/sortase system common in Gram-positive bacteria. This protein occurs in a species if and only if a transmembrane histidine kinase (TIGR02916) and a DNA-binding response regulator (TIGR02915) also occur. The present of tetratricopeptide repeats (TPR) suggests protein-protein interaction, possibly for the regulation of PEP-CTERM protein expression, since many PEP-CTERM proteins in these genomes are preceded by a proposed DNA binding site for the response regulator. |
| 274350 | PEP_TPR_lipo | 1.39e-31 | 51 | 461 | 469 | 877 | putative PEP-CTERM system TPR-repeat lipoprotein. This protein family occurs in strictly within a subset of Gram-negative bacterial species with the proposed PEP-CTERM/exosortase system, analogous to the LPXTG/sortase system common in Gram-positive bacteria. This protein occurs in a species if and only if a transmembrane histidine kinase (TIGR02916) and a DNA-binding response regulator (TIGR02915) also occur. The present of tetratricopeptide repeats (TPR) suggests protein-protein interaction, possibly for the regulation of PEP-CTERM protein expression, since many PEP-CTERM proteins in these genomes are preceded by a proposed DNA binding site for the response regulator. |
| 274350 | PEP_TPR_lipo | 2.44e-30 | 59 | 452 | 34 | 462 | putative PEP-CTERM system TPR-repeat lipoprotein. This protein family occurs in strictly within a subset of Gram-negative bacterial species with the proposed PEP-CTERM/exosortase system, analogous to the LPXTG/sortase system common in Gram-positive bacteria. This protein occurs in a species if and only if a transmembrane histidine kinase (TIGR02916) and a DNA-binding response regulator (TIGR02915) also occur. The present of tetratricopeptide repeats (TPR) suggests protein-protein interaction, possibly for the regulation of PEP-CTERM protein expression, since many PEP-CTERM proteins in these genomes are preceded by a proposed DNA binding site for the response regulator. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 16 | 994 | 32 | 1010 | |
| 2.61e-235 | 461 | 927 | 486 | 951 | |
| 4.30e-162 | 463 | 928 | 518 | 987 | |
| 9.13e-162 | 463 | 928 | 508 | 977 | |
| 9.13e-162 | 463 | 928 | 508 | 977 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.11e-128 | 463 | 922 | 147 | 715 | The human O-GlcNAc transferase in complex with a thiol-linked bisubstrate inhibitor [Homo sapiens] |
|
| 7.31e-128 | 463 | 922 | 148 | 716 | The human O-GlcNAc transferase in complex with a bisubstrate inhibitor [Homo sapiens] |
|
| 8.40e-128 | 463 | 922 | 153 | 721 | Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_B Chain B, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_D Chain D, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE4_A Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3PE4_C Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3TAX_A A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],3TAX_C A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],4AY5_A Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_B Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_C Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_D Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY6_A Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_B Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_C Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_D Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4CDR_A Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_B Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_C Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_D Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4GYW_A Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYW_C Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYY_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GYY_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GZ3_A Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ3_C Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ5_A Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_B Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_C Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_D Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ6_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_B Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_D Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4N39_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) [Homo sapiens],4N3A_A Crystal Structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (1-26)E10A [Homo sapiens],4N3B_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26)E10Q and UDP-5SGlcNAc [Homo sapiens],4N3C_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26) and UDP-GlcNAc [Homo sapiens],4XI9_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XIF_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],5BNW_A The active site of O-GlcNAc transferase imposes constraints on substrate sequence [Homo sapiens],5C1D_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RB2L) [Homo sapiens],5VIE_A Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIE_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIF_A Electrophilic probes for deciphering substrate recognition by O-GlcNAc transferase [Homo sapiens],6E37_A O-GlcNAc Transferase in complex with covalent inhibitor [Homo sapiens],6MA1_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 4a [Homo sapiens],6MA2_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor ent-1a [Homo sapiens],6MA3_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 2a [Homo sapiens],6MA4_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 3a [Homo sapiens],6MA5_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 1a [Homo sapiens] |
|
| 9.38e-128 | 463 | 922 | 152 | 720 | Chain AAA, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens] |
|
| 1.71e-127 | 463 | 922 | 174 | 742 | Human OGT in complex with UDP and fused substrate peptide (HCF1) [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.19e-158 | 463 | 928 | 503 | 972 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SEC OS=Arabidopsis thaliana OX=3702 GN=SEC PE=1 SV=1 |
|
| 1.10e-124 | 463 | 922 | 471 | 1039 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Mus musculus OX=10090 GN=Ogt PE=1 SV=2 |
|
| 8.01e-124 | 463 | 922 | 471 | 1039 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Oryctolagus cuniculus OX=9986 GN=OGT PE=1 SV=2 |
|
| 1.12e-123 | 463 | 922 | 471 | 1039 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Homo sapiens OX=9606 GN=OGT PE=1 SV=3 |
|
| 3.01e-123 | 463 | 922 | 471 | 1039 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Sus scrofa OX=9823 GN=OGT PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
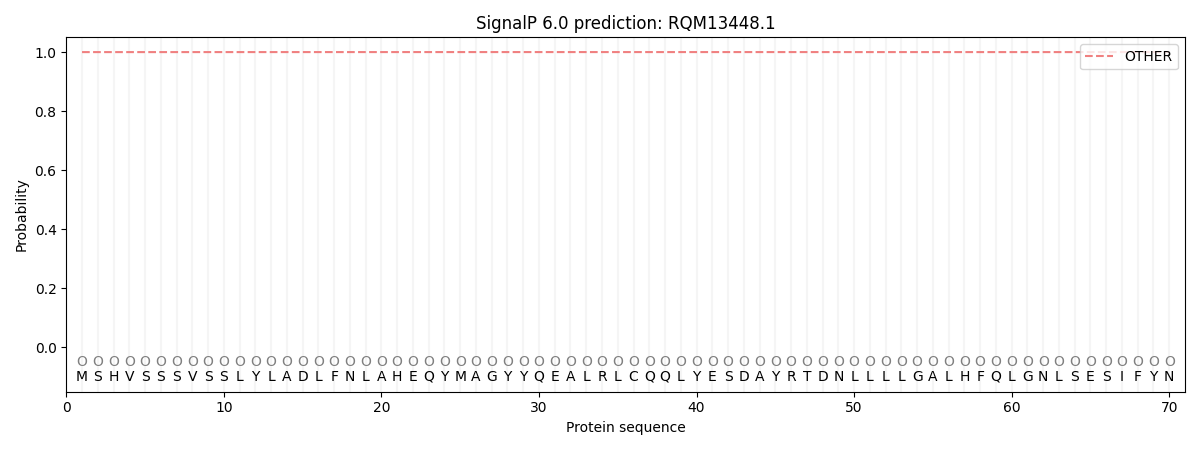
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000054 | 0.000003 |
