You are browsing environment: FUNGIDB
CAZyme Information: RL4_JR_01814-RA-p1
You are here: Home > Sequence: RL4_JR_01814-RA-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Raffaelea lauricola | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Ophiostomataceae; Raffaelea; Raffaelea lauricola | |||||||||||
| CAZyme ID | RL4_JR_01814-RA-p1 | |||||||||||
| CAZy Family | AA7 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.59:2 | - | - |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH71 | 45 | 432 | 8.1e-125 | 0.9786666666666667 |
| CBM24 | 473 | 547 | 2.1e-27 | 0.9868421052631579 |
| CBM24 | 566 | 636 | 5.3e-18 | 0.9605263157894737 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 397634 | Glyco_hydro_71 | 2.72e-136 | 22 | 431 | 1 | 372 | Glycosyl hydrolase family 71. Family of alpha-1,3-glucanases. |
| 211418 | GH71 | 5.67e-106 | 22 | 316 | 7 | 280 | Glycoside hydrolase family 71. This family of glycoside hydrolases 71 (following the CAZY nomenclature) function as alpha-1,3-glucanases (mutanases, EC 3.2.1.59). They appear to have an endo-hydrolytic mode of enzymatic activity and bacterial members are investigated as candidates for the development of dental caries treatments.The member from fission yeast, endo-alpha-1,3-glucanase Agn1p, plays a vital role in daughter cell separation, while Agn2p has been associated with endolysis of the ascus wall. |
| 238861 | SEST_like | 1.41e-42 | 911 | 1168 | 3 | 241 | SEST_like. A family of secreted SGNH-hydrolases similar to Streptomyces scabies esterase (SEST), a causal agent of the potato scab disease, which hydrolyzes a specific ester bond in suberin, a plant lipid. The tertiary fold of this enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles two of the three components of typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxylic acid. |
| 211414 | GH99_GH71_like | 1.62e-36 | 60 | 314 | 17 | 284 | Glycoside hydrolase families 71, 99, and related domains. This superfamily of glycoside hydrolases contains families GH71 and GH99 (following the CAZY nomenclature), as well as other members with undefined function and specificity. |
| 199914 | M35_deuterolysin_like | 9.91e-13 | 652 | 819 | 1 | 150 | Peptidase M35 domain of deuterolysins and related proteins. This family M35 Zn2+-metallopeptidase extracellular domain is found in fungal deutrolysins (acid metalloproteinase, neutral proteinase II), including some well-characterized metallopeptidase domains in Aspergillus oryzae (NpII), Aspergillus fumigatus (MEP20), Penicillium roqueforti (protease II) and Emericella nidulans (PepJ peptidase). The neutral proteinase II from Aspergillus oryzae (NpII) unfolds reversibly upon incubation at higher temperatures, and loss in activity is mainly due to autoproteolysis. MEP20 is encoded by the mepB gene, which appears to be associated with the cytoplasmic degradation of small peptides. PepJ peptidase is a thermostable enzyme released under carbon starvation. Most members of this family contain a unique zinc-binding motif (the aspzincin motif), defined by the HExxH + D motif where an aspartic acid is the third zinc ligand and is found in a GTXDXXYG or similar motif C-terminal to the His zinc ligands. The aspzincin motif is poorly conserved in one subgroup, that includes Asp f2, a major allergen from Aspergillus fumigatus. This subgroup in addition lacks the key conserved Tyr residue which acts as a proton donor during catalysis, and no protease activity has been detected to date for Asp f2. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 15 | 1300 | 18 | 1306 | |
| 1.52e-192 | 3 | 613 | 5 | 596 | |
| 1.66e-165 | 31 | 615 | 2 | 590 | |
| 4.96e-162 | 17 | 803 | 19 | 797 | |
| 2.37e-158 | 45 | 526 | 2 | 473 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.62e-18 | 910 | 1149 | 4 | 199 | Crystal structure of phospholipase A1 from Streptomyces albidoflavus NA297 [Streptomyces albidoflavus] |
|
| 7.51e-14 | 911 | 1149 | 4 | 198 | Crystal structure of extracelular lipase from Streptomyces rimosus at 1.7A resolution [Streptomyces rimosus],5MAL_B Crystal structure of extracelular lipase from Streptomyces rimosus at 1.7A resolution [Streptomyces rimosus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.23e-49 | 22 | 461 | 38 | 436 | Mutanase Pc12g07500 OS=Penicillium rubens (strain ATCC 28089 / DSM 1075 / NRRL 1951 / Wisconsin 54-1255) OX=500485 GN=PCH_Pc12g07500 PE=1 SV=1 |
|
| 9.95e-41 | 45 | 335 | 30 | 321 | Glucan endo-1,3-alpha-glucosidase agn1 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=agn1 PE=1 SV=2 |
|
| 1.44e-22 | 48 | 323 | 22 | 304 | Ascus wall endo-1,3-alpha-glucanase OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=agn2 PE=1 SV=2 |
|
| 6.82e-13 | 911 | 1149 | 38 | 232 | Lipase OS=Streptomyces rimosus OX=1927 PE=1 SV=1 |
|
| 6.82e-13 | 909 | 1075 | 36 | 184 | Lipase 1 OS=Streptomyces coelicolor (strain ATCC BAA-471 / A3(2) / M145) OX=100226 GN=SCO1725 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
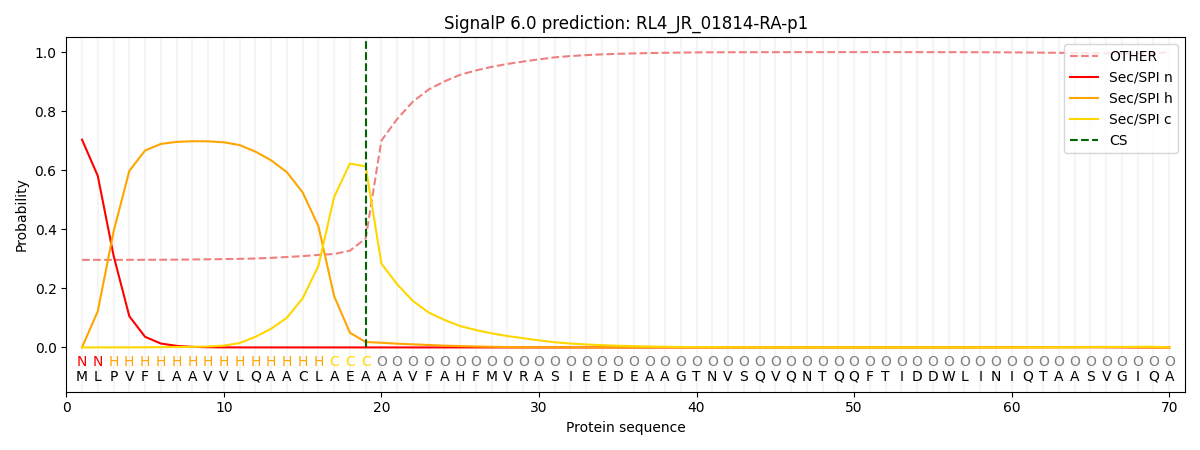
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.315969 | 0.684006 | CS pos: 19-20. Pr: 0.6129 |
