You are browsing environment: FUNGIDB
CAZyme Information: QYA_145794T0-p1
You are here: Home > Sequence: QYA_145794T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Mucor lusitanicus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Mucoromycota; Mucoromycetes; ; Mucoraceae; Mucor; Mucor lusitanicus | |||||||||||
| CAZyme ID | QYA_145794T0-p1 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | Chitin synthase [Source:UniProtKB/TrEMBL;Acc:A0A168JLF3] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.16:11 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 1200 | 1708 | 2.8e-240 | 0.9962049335863378 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 276845 | MYSc_Myo17 | 0.0 | 26 | 762 | 1 | 647 | class XVII myosin, motor domain. This fungal myosin which is also known as chitin synthase uses its motor domain to tether its vesicular cargo to peripheral actin. It works in opposition to dynein, contributing to the retention of Mcs1 vesicles at the site of cell growth and increasing vesicle fusion necessary for polarized growth. Class 17 myosins consist of a N-terminal myosin motor domain with Cyt-b5, chitin synthase 2, and a DEK_C domains at it C-terminus. The chitin synthase region contains several transmembrane domains by which myosin 17 is thought to bind secretory vesicles. The catalytic (head) domain has ATPase activity and belongs to the larger group of P-loop NTPases. Myosins are actin-dependent molecular motors that play important roles in muscle contraction, cell motility, and organelle transport. The head domain is a molecular motor, which utilizes ATP hydrolysis to generate directed movement toward the plus end along actin filaments. A cyclical interaction between myosin and actin provides the driving force. Rates of ATP hydrolysis and consequently the speed of movement along actin filaments vary widely, from about 0.04 micrometer per second for myosin I to 4.5 micrometer per second for myosin II in skeletal muscle. Myosin II moves in discrete steps about 5-10 nm long and generates 1-5 piconewtons of force. Upon ATP binding, the myosin head dissociates from an actin filament. ATP hydrolysis causes the head to pivot and associate with a new actin subunit. The release of Pi causes the head to pivot and move the filament (power stroke). Release of ADP completes the cycle. CyMoBase classifications were used to confirm and identify the myosins in this hierarchy. |
| 367353 | Chitin_synth_2 | 0.0 | 1199 | 1708 | 1 | 527 | Chitin synthase. Members of this family are fungal chitin synthase EC:2.4.1.16 enzymes. They catalyze chitin synthesis as follows: UDP-N-acetyl-D-glucosamine + {(1,4)-(N-acetyl-beta-D-glucosaminyl)}(N) <=> UDP + {(1,4)-(N-acetyl-beta-D-glucosaminyl)}(N+1). |
| 276950 | MYSc | 5.30e-132 | 35 | 753 | 7 | 633 | Myosin motor domain superfamily. Myosin motor domain. The catalytic (head) domain has ATPase activity and belongs to the larger group of P-loop NTPases. Myosins are actin-dependent molecular motors that play important roles in muscle contraction, cell motility, and organelle transport. The head domain is a molecular motor, which utilizes ATP hydrolysis to generate directed movement toward the plus end along actin filaments. A cyclical interaction between myosin and actin provides the driving force. Rates of ATP hydrolysis and consequently the speed of movement along actin filaments vary widely, from about 0.04 micrometer per second for myosin I to 4.5 micrometer per second for myosin II in skeletal muscle. Myosin II moves in discrete steps about 5-10 nm long and generates 1-5 piconewtons of force. Upon ATP binding, the myosin head dissociates from an actin filament. ATP hydrolysis causes the head to pivot and associate with a new actin subunit. The release of Pi causes the head to pivot and move the filament (power stroke). Release of ADP completes the cycle. CyMoBase classifications were used to confirm and identify the myosins in this hierarchy. |
| 214580 | MYSc | 4.09e-127 | 15 | 762 | 7 | 674 | Myosin. Large ATPases. ATPase; molecular motor. Muscle contraction consists of a cyclical interaction between myosin and actin. The core of the myosin structure is similar in fold to that of kinesin. |
| 276849 | MYSc_Myo22 | 7.98e-110 | 35 | 752 | 7 | 660 | class XXII myosin, motor domain. These myosins possess an extended neck with multiple IQ motifs such as found in class V, VIII, XI, and XIII myosins. These myosins are defined by two tandem MyTH4 and FERM domains. The apicomplexan, but not diatom myosins contain 4-6 WD40 repeats near the end of the C-terminal tail which suggests a possible function of these myosins in signal transduction and transcriptional regulation. The catalytic (head) domain has ATPase activity and belongs to the larger group of P-loop NTPases. Myosins are actin-dependent molecular motors that play important roles in muscle contraction, cell motility, and organelle transport. The head domain is a molecular motor, which utilizes ATP hydrolysis to generate directed movement toward the plus end along actin filaments. A cyclical interaction between myosin and actin provides the driving force. Rates of ATP hydrolysis and consequently the speed of movement along actin filaments vary widely, from about 0.04 micrometer per second for myosin I to 4.5 micrometer per second for myosin II in skeletal muscle. Myosin II moves in discrete steps about 5-10 nm long and generates 1-5 piconewtons of force. Upon ATP binding, the myosin head dissociates from an actin filament. ATP hydrolysis causes the head to pivot and associate with a new actin subunit. The release of Pi causes the head to pivot and move the filament (power stroke). Release of ADP completes the cycle. CyMoBase classifications were used to confirm and identify the myosins in this hierarchy. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 16 | 1709 | 11 | 1689 | |
| 0.0 | 16 | 1913 | 14 | 1891 | |
| 0.0 | 16 | 1913 | 13 | 1883 | |
| 0.0 | 134 | 1913 | 5 | 1618 | |
| 0.0 | 3 | 1913 | 2 | 1906 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.00e-65 | 35 | 761 | 83 | 735 | Crystal structure of myosin X motor domain in pre-powerstroke state [Homo sapiens],5I0H_B Crystal structure of myosin X motor domain in pre-powerstroke state [Homo sapiens] |
|
| 1.28e-65 | 35 | 777 | 81 | 745 | Crystal structure of myosin X motor domain with 2IQ motifs in pre-powerstroke state [Homo sapiens],5I0I_B Crystal structure of myosin X motor domain with 2IQ motifs in pre-powerstroke state [Homo sapiens] |
|
| 7.72e-65 | 35 | 761 | 81 | 733 | Rigor myosin X co-complexed with an actin filament [Homo sapiens] |
|
| 1.74e-64 | 16 | 761 | 4 | 675 | Chain D, Isoform 3 of Unconventional myosin-XV [Mus musculus] |
|
| 3.15e-64 | 16 | 761 | 4 | 675 | Chain D, Isoform 3 of Unconventional myosin-XV [Mus musculus],7RB8_D Chain D, Isoform 3 of Unconventional myosin-XV [Mus musculus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 6 | 1913 | 3 | 1929 | Chitin synthase 5 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=CHS5 PE=2 SV=1 |
|
| 0.0 | 16 | 1914 | 7 | 2003 | Chitin synthase 8 OS=Ustilago maydis (strain 521 / FGSC 9021) OX=237631 GN=CHS8 PE=3 SV=1 |
|
| 8.15e-304 | 873 | 1711 | 65 | 896 | Chitin synthase 6 OS=Ustilago maydis (strain 521 / FGSC 9021) OX=237631 GN=CHS6 PE=3 SV=2 |
|
| 7.41e-277 | 875 | 1757 | 160 | 1036 | Chitin synthase 4 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=CHS4 PE=2 SV=2 |
|
| 1.22e-144 | 1226 | 1708 | 686 | 1184 | Chitin synthase 4 OS=Magnaporthe oryzae (strain 70-15 / ATCC MYA-4617 / FGSC 8958) OX=242507 GN=CHS4 PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000049 | 0.000000 |
TMHMM Annotations download full data without filtering help
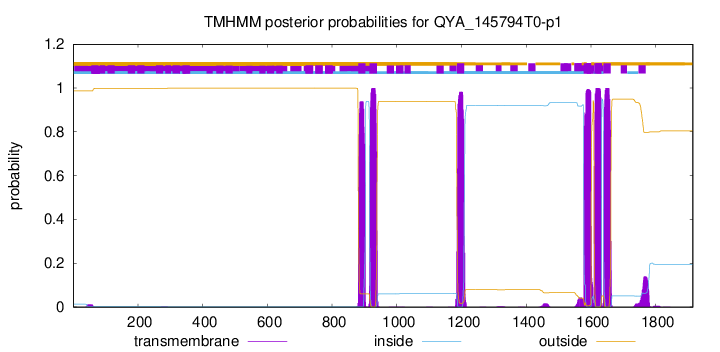
| Start | End |
|---|---|
| 881 | 903 |
| 916 | 938 |
| 1186 | 1208 |
| 1578 | 1600 |
| 1610 | 1632 |
| 1639 | 1661 |
