You are browsing environment: FUNGIDB
CAZyme Information: QRD89368.1
You are here: Home > Sequence: QRD89368.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus flavus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus flavus | |||||||||||
| CAZyme ID | QRD89368.1 | |||||||||||
| CAZy Family | GH32 | |||||||||||
| CAZyme Description | putative endoglycoceramidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH5 | 61 | 392 | 8.8e-135 | 0.9969135802469136 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 408348 | Glyco_hydro_5_C | 1.82e-16 | 413 | 482 | 1 | 69 | Glycoside hydrolase family 5 C-terminal domain. This is the C-terminal domain of endo-glycoceramidase II (EGC), a membrane-associated family 5 glycosidase pfam00150. The C-terminal domain assumes a beta-sandwich fold, which resembles that of many carbohydrate-binding modules. |
| 395098 | Cellulase | 5.22e-13 | 89 | 392 | 27 | 268 | Cellulase (glycosyl hydrolase family 5). |
| 396834 | Glyco_hydro_42 | 3.64e-05 | 89 | 256 | 13 | 138 | Beta-galactosidase. This group of beta-galactosidase enzymes belong to the glycosyl hydrolase 42 family. The enzyme catalyzes the hydrolysis of terminal, non-reducing terminal beta-D-galactosidase residues. |
| 224786 | GanA | 1.07e-04 | 72 | 263 | 23 | 199 | Beta-galactosidase GanA [Carbohydrate transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 889 | 1 | 889 | |
| 0.0 | 1 | 483 | 1 | 483 | |
| 0.0 | 1 | 483 | 1 | 483 | |
| 0.0 | 1 | 483 | 1 | 483 | |
| 0.0 | 1 | 483 | 1 | 483 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.21e-27 | 37 | 476 | 36 | 455 | Chain A, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S],5CCU_B Chain B, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S] |
|
| 7.06e-27 | 37 | 476 | 36 | 455 | Chain A, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S],5J14_B Chain B, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S],5J7Z_A Chain A, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S],5J7Z_B Chain B, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S] |
|
| 1.48e-22 | 54 | 465 | 39 | 448 | Endo-glycoceramidase II from Rhodococcus sp. [Rhodococcus sp.],2OSW_B Endo-glycoceramidase II from Rhodococcus sp. [Rhodococcus sp.],2OYK_A Endo-glycoceramidase II from Rhodococcus sp.: cellobiose-like isofagomine complex [Rhodococcus sp.],2OYK_B Endo-glycoceramidase II from Rhodococcus sp.: cellobiose-like isofagomine complex [Rhodococcus sp.],2OYL_A Endo-glycoceramidase II from Rhodococcus sp.: cellobiose-like imidazole complex [Rhodococcus sp.],2OYL_B Endo-glycoceramidase II from Rhodococcus sp.: cellobiose-like imidazole complex [Rhodococcus sp.],2OYM_A Endo-glycoceramidase II from Rhodococcus sp.: five-membered iminocyclitol complex [Rhodococcus sp.],2OYM_B Endo-glycoceramidase II from Rhodococcus sp.: five-membered iminocyclitol complex [Rhodococcus sp.] |
|
| 2.63e-22 | 54 | 465 | 39 | 448 | Chain A, Endoglycoceramidase II [Rhodococcus sp.] |
|
| 1.58e-14 | 38 | 421 | 18 | 415 | Chain A, Putative secreted endoglycosylceramidase [Rhodococcus hoagii 103S] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.25e-84 | 40 | 472 | 29 | 473 | Endoglycoceramidase OS=Cyanea nozakii OX=135523 PE=1 SV=1 |
|
| 3.45e-78 | 45 | 478 | 25 | 461 | Endoglycoceramidase OS=Hydra vulgaris OX=6087 PE=1 SV=1 |
|
| 1.33e-44 | 516 | 886 | 71 | 461 | Uncharacterized protein AN5342 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=AN5342 PE=1 SV=1 |
|
| 2.50e-26 | 42 | 476 | 33 | 447 | Endoglycoceramidase I OS=Rhodococcus hoagii (strain 103S) OX=685727 GN=REQ_38260 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
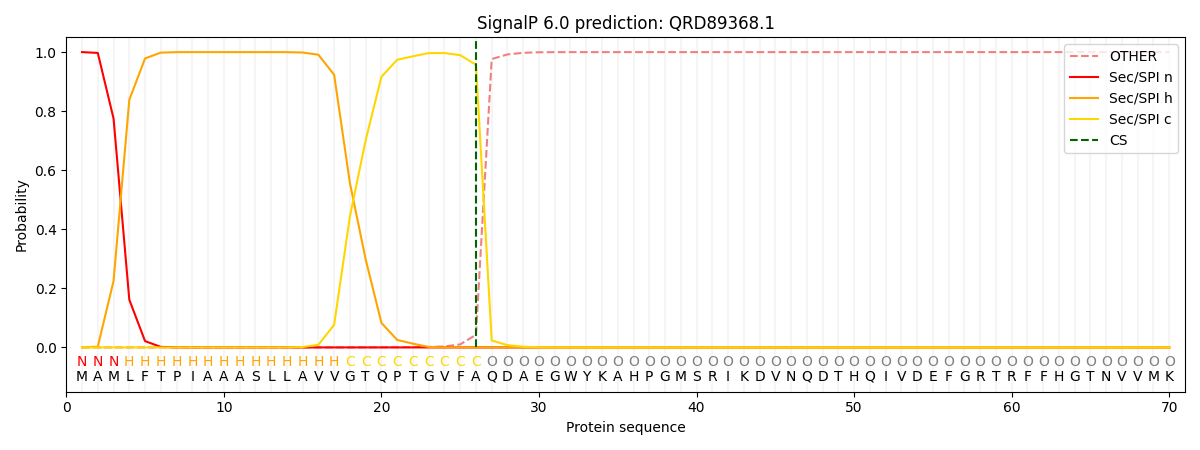
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000305 | 0.999668 | CS pos: 26-27. Pr: 0.9572 |
