You are browsing environment: FUNGIDB
CAZyme Information: QRD81157.1
You are here: Home > Sequence: QRD81157.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus flavus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus flavus | |||||||||||
| CAZyme ID | QRD81157.1 | |||||||||||
| CAZy Family | AA1 | |||||||||||
| CAZyme Description | putative laccase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 36823; End:38648 Strand: - | |||||||||||
Full Sequence Download help
| MMGLLCPRLR GILHTLSLVL GLVTATQGHL VLHDDSFQPD HILRVTAQDV NQACMDRYSV | 60 |
| LINGSLPGPQ LNIQEGKVNW IRVYNDMEDL NVTMHWHGLS AFTAPFSDGT PMASQWPIPP | 120 |
| GHFFDYEVRP EVGYAGTYFY HSHVGFQALT AWGALIVESA QPSPYQYDEE RIIALSDFFT | 180 |
| KTDEEIENGL TSTNFTWSGE TSAVLVNGQG RLATNATGSC KLAAISVEPG KTYRLRFIGA | 240 |
| TALSFVSISL ESHDVLEIIE ADGHYTKPVN TSYLQISSGQ RYSVLLKAKT EAELQQAKSR | 300 |
| QFYFQLTTMG RPTVLTTFAV LEYPSPTTTD LITVPVTPPL PVANITYGWL DYTLEPYYPD | 360 |
| LDFPTVEEVT RRIIINVHQN ISDRTVWLQN GYDWVETFPK SPYLVDIYAG TLDLDASYKR | 420 |
| AIASGYAFDN QTRLFPAKMG EVLEIVWQNQ GAVSNGGVEN HPFHAHGRHF YDIGGGDGLY | 480 |
| NLTENEARLK GTHPVIRDTT MLYAYRKTTT ALEPSGWRAW RIRVTAAGVW MVHCHVLQHM | 540 |
| LMGMQTAFAF GDQTAIKAQS GTPAEGYLTY GGSAYGNVTH FPPVKHFFN | 589 |
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 50 | 545 | 1.5e-92 | 0.994413407821229 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 132431 | ascorbOXfungal | 0.0 | 31 | 557 | 1 | 538 | L-ascorbate oxidase, fungal type. This model describes a family of fungal ascorbate oxidases, within a larger family of multicopper oxidases that also includes plant ascorbate oxidases (TIGR03388), plant laccases and laccase-like proteins (TIGR03389), and related proteins. The member from Acremonium sp. HI-25 is characterized. |
| 259962 | CuRO_3_AAO_like_2 | 7.59e-88 | 368 | 551 | 1 | 188 | The third cupredoxin domain of Ascorbate oxidase homologs. This family includes fungal proteins with similarity to ascorbate oxidase. Ascorbate oxidase catalyzes the oxidation of ascorbic acid to dehydroascorbic acid. It can detect levels of ascorbic acid and eliminate it. The biological function of ascorbate oxidase is still not clear. Ascorbate oxidase belongs to multicopper oxidase (MCO) family which couple oxidation of substrates with reduction of dioxygen to water. MCOs are capable of oxidizing a vast range of substrates, varying from aromatic compounds to inorganic compounds such as metals. Although the members of this family have diverse functions, majority of them have three cupredoxin domain repeats. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 3 of 3-domain MCOs contains the Type 1 (T1) copper binding site and part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 274555 | ascorbase | 1.43e-87 | 54 | 551 | 17 | 524 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
| 215324 | PLN02604 | 4.15e-76 | 54 | 551 | 40 | 547 | oxidoreductase |
| 177843 | PLN02191 | 1.17e-72 | 54 | 551 | 39 | 547 | L-ascorbate oxidase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QRD81157.1|AA1 | 0.0 | 1 | 589 | 1 | 589 |
| QMW38925.1|AA1 | 0.0 | 2 | 589 | 1 | 588 |
| QMW26845.1|AA1 | 0.0 | 2 | 589 | 1 | 588 |
| UDD54641.1|AA1 | 0.0 | 2 | 589 | 1 | 588 |
| BCS19632.1|AA1 | 1.17e-301 | 9 | 587 | 1 | 576 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1AOZ_A | 3.39e-58 | 54 | 551 | 19 | 524 | Refined Crystal Structure Of Ascorbate Oxidase At 1.9 Angstroms Resolution [Cucurbita pepo var. melopepo],1AOZ_B Refined Crystal Structure Of Ascorbate Oxidase At 1.9 Angstroms Resolution [Cucurbita pepo var. melopepo],1ASO_A X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASO_B X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASP_A X-ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-forms [Cucurbita pepo var. melopepo],1ASP_B X-ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-forms [Cucurbita pepo var. melopepo],1ASQ_A X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASQ_B X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo] |
| 6KLG_A | 4.70e-39 | 45 | 548 | 10 | 528 | Crystal Structure of the Zea Mays laccase 3 [Zea mays],6KLI_A Crystal Structure of the Zea Mays laccase 3 complexed with sinapyl [Zea mays],6KLJ_A Crystal Structure of the Zea Mays laccase 3 complexed with coniferyl [Zea mays] |
| 5Z1X_A | 3.79e-32 | 43 | 565 | 8 | 479 | Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena],5Z1X_B Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena],5Z22_A Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena] |
| 1HFU_A | 2.55e-31 | 43 | 549 | 9 | 467 | Chain A, LACCASE 1 [Coprinopsis cinerea] |
| 1A65_A | 2.58e-31 | 43 | 549 | 9 | 467 | Chain A, Laccase [Coprinopsis cinerea] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| sp|G2QFD0|LMCO1_MYCTT | 1.93e-174 | 12 | 589 | 2 | 632 | Laccase-like multicopper oxidase 1 OS=Myceliophthora thermophila (strain ATCC 42464 / BCRC 31852 / DSM 1799) OX=573729 GN=LMCO1 PE=1 SV=1 |
| sp|I1RF64|AURL2_GIBZE | 1.37e-170 | 32 | 589 | 21 | 598 | Multicopper oxidase aurL2 OS=Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) OX=229533 GN=aurL2 PE=2 SV=1 |
| sp|Q0D1P3|TERE_ASPTN | 3.11e-132 | 94 | 589 | 1 | 528 | Multicopper oxidase terE OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=terE PE=1 SV=1 |
| sp|P14133|ASO_CUCSA | 9.56e-61 | 54 | 551 | 54 | 560 | L-ascorbate oxidase OS=Cucumis sativus OX=3659 PE=1 SV=1 |
| sp|Q40588|ASO_TOBAC | 1.09e-58 | 62 | 551 | 54 | 551 | L-ascorbate oxidase OS=Nicotiana tabacum OX=4097 GN=AAO PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
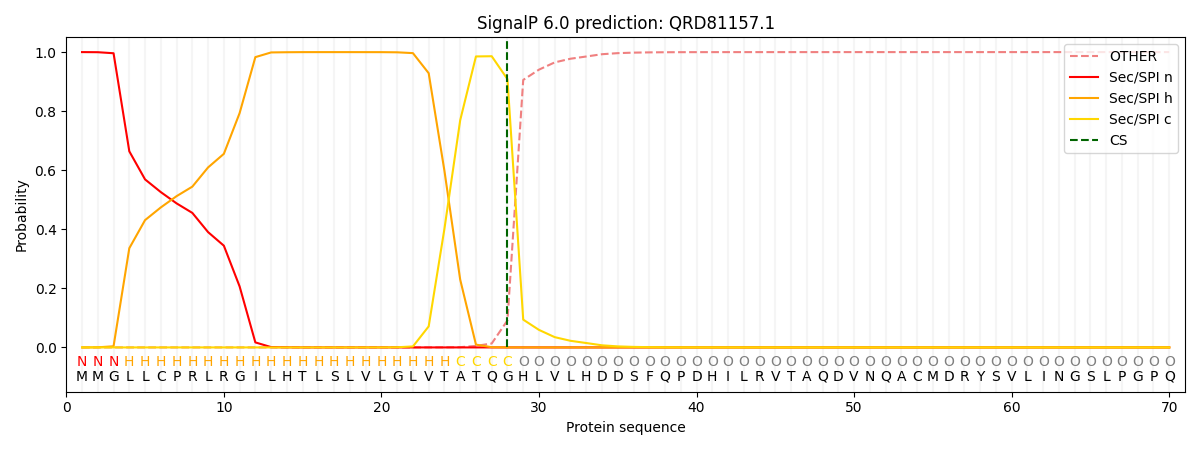
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000367 | 0.999625 | CS pos: 28-29. Pr: 0.9084 |
