You are browsing environment: FUNGIDB
CAZyme Information: QRD03154.1
You are here: Home > Sequence: QRD03154.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parastagonospora nodorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Phaeosphaeriaceae; Parastagonospora; Parastagonospora nodorum | |||||||||||
| CAZyme ID | QRD03154.1 | |||||||||||
| CAZy Family | GH47 | |||||||||||
| CAZyme Description | FAD-binding PCMH-type domain-containing protein [Source:UniProtKB/TrEMBL;Acc:A0A7U2I663] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 56 | 491 | 7.6e-57 | 0.9716157205240175 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 223354 | GlcD | 4.01e-14 | 57 | 260 | 26 | 234 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 396238 | FAD_binding_4 | 1.66e-12 | 63 | 199 | 1 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 178402 | PLN02805 | 2.53e-04 | 59 | 226 | 130 | 303 | D-lactate dehydrogenase [cytochrome] |
| 369658 | BBE | 0.002 | 455 | 490 | 2 | 38 | Berberine and berberine like. This domain is found in the berberine bridge and berberine bridge- like enzymes which are involved in the biosynthesis of numerous isoquinoline alkaloids. They catalyze the transformation of the N-methyl group of (S)-reticuline into the C-8 berberine bridge carbon of (S)-scoulerine. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 9.65e-19 | 15 | 246 | 13 | 241 | |
| 6.28e-17 | 43 | 240 | 58 | 246 | |
| 8.17e-16 | 26 | 272 | 38 | 280 | |
| 7.99e-15 | 63 | 494 | 90 | 512 | |
| 8.88e-15 | 1 | 259 | 1 | 263 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.28e-15 | 60 | 250 | 45 | 232 | Physcomitrella patens BBE-like 1 variant D396N [Physcomitrium patens],6EO5_B Physcomitrella patens BBE-like 1 variant D396N [Physcomitrium patens] |
|
| 4.28e-15 | 60 | 250 | 45 | 232 | Physcomitrella patens BBE-like 1 wild-type [Physcomitrium patens],6EO4_B Physcomitrella patens BBE-like 1 wild-type [Physcomitrium patens] |
|
| 5.39e-15 | 27 | 224 | 10 | 205 | The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_B The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_C The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_D The crystal structure of EncM T139V mutant [Streptomyces maritimus] |
|
| 8.00e-15 | 15 | 259 | 10 | 255 | Xylooligosaccharide oxidase from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],5L6F_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylobiose [Thermothelomyces thermophilus ATCC 42464],5L6G_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylose [Thermothelomyces thermophilus ATCC 42464] |
|
| 9.54e-15 | 27 | 224 | 10 | 205 | The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_B The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_C The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_D The crystal structure of EncM V135M mutant [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.89e-66 | 25 | 500 | 8 | 476 | FAD-dependent monooxygenase sdcF OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=sdcF PE=1 SV=1 |
|
| 2.50e-63 | 19 | 500 | 38 | 513 | FAD-dependent monooxygenase drtC OS=Aspergillus calidoustus OX=454130 GN=drtC PE=1 SV=1 |
|
| 3.53e-46 | 9 | 500 | 17 | 517 | FAD-dependent monooxygenase tpcD OS=Cochliobolus heterostrophus (strain C5 / ATCC 48332 / race O) OX=701091 GN=tpcD PE=1 SV=1 |
|
| 1.55e-44 | 26 | 500 | 32 | 507 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti OX=5082 GN=prx3 PE=3 SV=1 |
|
| 1.55e-44 | 26 | 500 | 32 | 507 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti (strain FM164) OX=1365484 GN=prx3 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
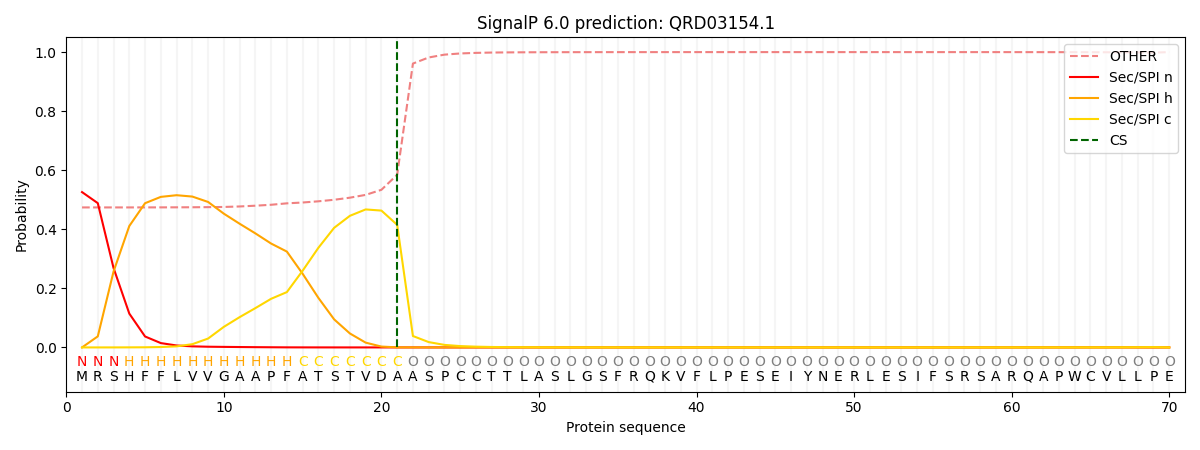
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.492111 | 0.507876 | CS pos: 21-22. Pr: 0.4151 |
