You are browsing environment: FUNGIDB
CAZyme Information: QRD01632.1
You are here: Home > Sequence: QRD01632.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
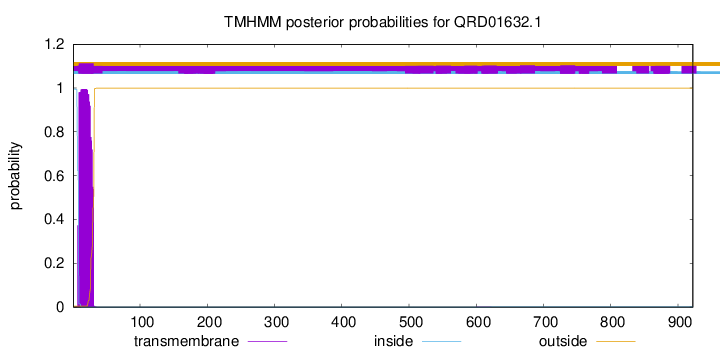
TMHMM annotations
Basic Information help
| Species | Parastagonospora nodorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Phaeosphaeriaceae; Parastagonospora; Parastagonospora nodorum | |||||||||||
| CAZyme ID | QRD01632.1 | |||||||||||
| CAZy Family | GH3 | |||||||||||
| CAZyme Description | alpha-1,2-Mannosidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.113:7 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH47 | 190 | 920 | 6.2e-176 | 0.9955156950672646 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396217 | Glyco_hydro_47 | 0.0 | 189 | 920 | 1 | 453 | Glycosyl hydrolase family 47. Members of this family are alpha-mannosidases that catalyze the hydrolysis of the terminal 1,2-linked alpha-D-mannose residues in the oligo-mannose oligosaccharide Man(9)(GlcNAc)(2). |
| 240427 | PTZ00470 | 2.69e-94 | 130 | 921 | 30 | 519 | glycoside hydrolase family 47 protein; Provisional |
| 397323 | Atrophin-1 | 0.004 | 74 | 159 | 177 | 255 | Atrophin-1 family. Atrophin-1 is the protein product of the dentatorubral-pallidoluysian atrophy (DRPLA) gene. DRPLA OMIM:125370 is a progressive neurodegenerative disorder. It is caused by the expansion of a CAG repeat in the DRPLA gene on chromosome 12p. This results in an extended polyglutamine region in atrophin-1, that is thought to confer toxicity to the protein, possibly through altering its interactions with other proteins. The expansion of a CAG repeat is also the underlying defect in six other neurodegenerative disorders, including Huntington's disease. One interaction of expanded polyglutamine repeats that is thought to be pathogenic is that with the short glutamine repeat in the transcriptional coactivator CREB binding protein, CBP. This interaction draws CBP away from its usual nuclear location to the expanded polyglutamine repeat protein aggregates that are characteristic of the polyglutamine neurodegenerative disorders. This interferes with CBP-mediated transcription and causes cytotoxicity. |
| 237057 | PRK12323 | 0.005 | 55 | 146 | 416 | 509 | DNA polymerase III subunit gamma/tau. |
| 275319 | predic_Ig_block | 0.006 | 96 | 165 | 75 | 141 | putative immunoglobulin-blocking virulence protein. Members of this family are putative virulence proteins of Mycoplasma and Ureaplasma species. Members share a region of sequence similarity (see TIGR04524) with protein M, a Mycoplasma genitalium protein that binds a conserved light chain region of IgG and blocks its protective function of antigen-specific binding. The seed alignment for this model includes an N-terminal signal-anchor domain and a proline-rich linker domain, and a C-terminal extension, in addition to the protein M-like domain recognized by TIGR04524. [Cellular processes, Pathogenesis] |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 922 | 1 | 922 | |
| 0.0 | 1 | 922 | 1 | 968 | |
| 0.0 | 1 | 922 | 1 | 910 | |
| 1.12e-234 | 123 | 922 | 148 | 978 | |
| 7.57e-234 | 134 | 922 | 162 | 995 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.08e-38 | 189 | 917 | 12 | 448 | Crystal structure of the class I human endoplasmic reticulum 1,2-alpha-mannosidase and Man9GlcNAc2-PA complex [Homo sapiens] |
|
| 1.21e-38 | 189 | 917 | 17 | 453 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase [Homo sapiens] |
|
| 1.25e-38 | 189 | 917 | 17 | 453 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With 1-Deoxymannojirimycin [Homo sapiens],1FO3_A Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With Kifunensine [Homo sapiens] |
|
| 4.22e-38 | 189 | 917 | 95 | 531 | Crystal Structure Of Human Class I alpha-1,2-Mannosidase In Complex With Thio-Disaccharide Substrate Analogue [Homo sapiens] |
|
| 5.12e-38 | 189 | 917 | 12 | 448 | Crystal structure of the class I human endoplasmic reticulum 1,2-alpha-mannosidase T688A mutant and Thio-disaccharide substrate analog complex [Homo sapiens],5KK7_B Crystal structure of the class I human endoplasmic reticulum 1,2-alpha-mannosidase T688A mutant and Thio-disaccharide substrate analog complex [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.04e-45 | 144 | 917 | 158 | 623 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IB OS=Homo sapiens OX=9606 GN=MAN1A2 PE=1 SV=1 |
|
| 3.70e-45 | 170 | 917 | 172 | 623 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IB OS=Mus musculus OX=10090 GN=Man1a2 PE=1 SV=1 |
|
| 5.92e-39 | 189 | 917 | 214 | 650 | Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase OS=Rattus norvegicus OX=10116 GN=Man1b1 PE=2 SV=2 |
|
| 1.07e-38 | 189 | 917 | 215 | 651 | Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase OS=Mus musculus OX=10090 GN=Man1b1 PE=1 SV=1 |
|
| 2.85e-38 | 182 | 918 | 197 | 642 | Mannosyl-oligosaccharide alpha-1,2-mannosidase IA OS=Spodoptera frugiperda OX=7108 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.999985 | 0.000041 |