You are browsing environment: FUNGIDB
CAZyme Information: QRD00806.1
You are here: Home > Sequence: QRD00806.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parastagonospora nodorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Phaeosphaeriaceae; Parastagonospora; Parastagonospora nodorum | |||||||||||
| CAZyme ID | QRD00806.1 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | Glycos_transf_1 domain-containing protein [Source:UniProtKB/TrEMBL;Acc:A0A7U2FD79] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.231:3 |
|---|
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 340823 | GT4_trehalose_phosphorylase | 5.94e-148 | 265 | 703 | 1 | 378 | trehalose phosphorylase and similar proteins. Trehalose phosphorylase (TP) reversibly catalyzes trehalose synthesis and degradation from alpha-glucose-1-phosphate (alpha-Glc-1-P) and glucose. The catalyzing activity includes the phosphorolysis of trehalose, which produce alpha-Glc-1-P and glucose, and the subsequent synthesis of trehalose. This family is most closely related to the GT4 family of glycosyltransferases. |
| 223515 | RfaB | 3.42e-16 | 480 | 705 | 182 | 372 | Glycosyltransferase involved in cell wall bisynthesis [Cell wall/membrane/envelope biogenesis]. |
| 340816 | Glycosyltransferase_GTB-type | 3.34e-14 | 466 | 634 | 81 | 235 | glycosyltransferase family 1 and related proteins with GTB topology. Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. The structures of the formed glycoconjugates are extremely diverse, reflecting a wide range of biological functions. The members of this family share a common GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. |
| 340831 | GT4_PimA-like | 5.98e-13 | 478 | 685 | 179 | 350 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| 395425 | Glycos_transf_1 | 4.62e-12 | 490 | 679 | 1 | 155 | Glycosyl transferases group 1. Mutations in this domain of PIGA lead to disease (Paroxysmal Nocturnal haemoglobinuria). Members of this family transfer activated sugars to a variety of substrates, including glycogen, Fructose-6-phosphate and lipopolysaccharides. Members of this family transfer UDP, ADP, GDP or CMP linked sugars. The eukaryotic glycogen synthases may be distant members of this family. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 750 | 1 | 750 | |
| 4.63e-220 | 1 | 742 | 1 | 707 | |
| 3.32e-210 | 44 | 745 | 49 | 720 | |
| 5.73e-210 | 47 | 742 | 54 | 702 | |
| 3.75e-195 | 32 | 742 | 39 | 697 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.62e-42 | 237 | 640 | 16 | 368 | Crystal structure of trehalose synthase TreT from P.horikoshi [Pyrococcus horikoshii],2X6Q_B Crystal structure of trehalose synthase TreT from P.horikoshi [Pyrococcus horikoshii],2X6R_A Crystal structure of trehalose synthase TreT from P.horikoshi produced by soaking in trehalose [Pyrococcus horikoshii],2X6R_B Crystal structure of trehalose synthase TreT from P.horikoshi produced by soaking in trehalose [Pyrococcus horikoshii] |
|
| 5.54e-41 | 237 | 640 | 16 | 368 | Crystal structure of trehalose synthase TreT mutant E326A from P. horikoshii in complex with UDPG [Pyrococcus horikoshii],2XA2_B Crystal structure of trehalose synthase TreT mutant E326A from P. horikoshii in complex with UDPG [Pyrococcus horikoshii],2XA9_A Crystal structure of trehalose synthase TreT mutant E326A from P. horikoshii in complex with UDPG [Pyrococcus horikoshii],2XA9_B Crystal structure of trehalose synthase TreT mutant E326A from P. horikoshii in complex with UDPG [Pyrococcus horikoshii],2XMP_A Crystal structure of trehalose synthase TreT mutant E326A from P. horishiki in complex with UDP [Pyrococcus horikoshii],2XMP_B Crystal structure of trehalose synthase TreT mutant E326A from P. horishiki in complex with UDP [Pyrococcus horikoshii] |
|
| 2.60e-40 | 237 | 640 | 16 | 368 | Crystal structure of trehalose synthase TreT from P.horikoshii (Seleno derivative) [Pyrococcus horikoshii],2XA1_B Crystal structure of trehalose synthase TreT from P.horikoshii (Seleno derivative) [Pyrococcus horikoshii] |
|
| 7.43e-35 | 237 | 635 | 2 | 345 | Chain AAA, Trehalose phosphorylase/synthase [Thermoproteus uzoniensis],6ZJ7_AAA Chain AAA, Trehalose phosphorylase/synthase [Thermoproteus uzoniensis 768-20],6ZJH_AAA Chain AAA, Trehalose phosphorylase/synthase [Thermoproteus uzoniensis 768-20],6ZMZ_AAA Chain AAA, Trehalose phosphorylase/synthase [Thermoproteus uzoniensis],6ZN1_AAA Chain AAA, Trehalose phosphorylase/synthase [Thermoproteus uzoniensis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.55e-173 | 62 | 745 | 57 | 735 | Trehalose phosphorylase OS=Pleurotus pulmonarius OX=28995 GN=TP PE=2 SV=1 |
|
| 1.92e-171 | 40 | 745 | 32 | 728 | Trehalose phosphorylase OS=Grifola frondosa OX=5627 PE=1 SV=1 |
|
| 6.75e-168 | 62 | 745 | 57 | 747 | Trehalose phosphorylase OS=Pleurotus sajor-caju OX=50053 GN=TP PE=1 SV=1 |
|
| 6.33e-42 | 237 | 653 | 14 | 390 | Trehalose synthase OS=Thermococcus litoralis (strain ATCC 51850 / DSM 5473 / JCM 8560 / NS-C) OX=523849 GN=treT PE=1 SV=1 |
|
| 6.33e-42 | 237 | 653 | 14 | 390 | Trehalose synthase OS=Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) OX=186497 GN=treT PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
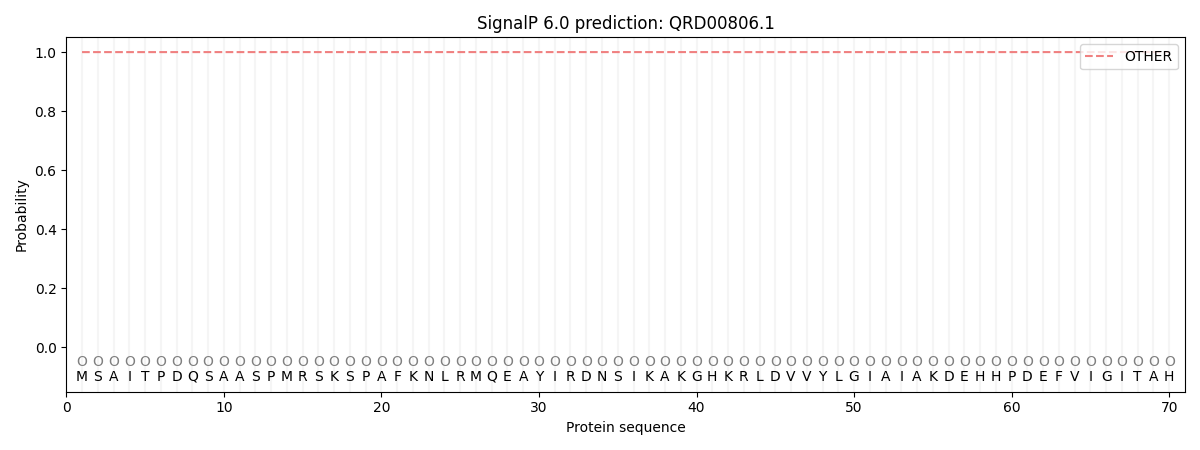
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000029 | 0.000001 |
