You are browsing environment: FUNGIDB
CAZyme Information: QRC95219.1
You are here: Home > Sequence: QRC95219.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parastagonospora nodorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Phaeosphaeriaceae; Parastagonospora; Parastagonospora nodorum | |||||||||||
| CAZyme ID | QRC95219.1 | |||||||||||
| CAZy Family | CBM508 | |||||||||||
| CAZyme Description | Integral membrane protein [Source:UniProtKB/TrEMBL;Acc:Q0UZ92] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT109 | 3 | 411 | 2.1e-130 | 0.9708029197080292 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 409190 | PGAP4-like_fungal | 0.0 | 4 | 384 | 2 | 374 | uncharacterized fungal proteins similar to Post-GPI attachment to proteins factor 4. This subfamily contains uncharacterized fungal proteins with similarity to animal post-GPI attachment to proteins factor 4 (PGAP4), also known as post-GPI attachment to proteins GalNAc transferase 4 or transmembrane protein 246 (TMEM246). PGAP4 has been shown to be a Golgi-resident GPI-GalNAc transferase. Many eukaryotic proteins are anchored to the cell surface through glycolipid glycosylphosphatidylinositol (GPI). GPIs have a conserved core but exhibit diverse N-acetylgalactosamine (GalNAc) modifications. PGAP4 knockout cells lose GPI-GalNAc structures. PGAP4 is most likely involved in the initial steps of GPI-GalNAc biosynthesis. In contrast to other Golgi glycotransferases, it contains three transmembrane domains. Proteins from this subfamily contain the putative catalytic site of PGAP4 and may have similar activities. |
| 409189 | PGAP4-like | 1.40e-45 | 10 | 382 | 3 | 361 | Post-GPI attachment to proteins factor 4 and similar proteins. This family includes post-GPI attachment to proteins factor 4 (PGAP4), also known as post-GPI attachment to proteins GalNAc transferase 4 or transmembrane protein 246 (TMEM246). PGAP4 has been shown to be a Golgi-resident GPI-GalNAc transferase. Many eukaryotic proteins are anchored to the cell surface through glycolipid glycosylphosphatidylinositol (GPI). GPIs have a conserved core but exhibit diverse N-acetylgalactosamine (GalNAc) modifications. PGAP4 knockout cells lose GPI-GalNAc structures. PGAP4 is most likely involved in the initial steps of GPI-GalNAc biosynthesis. In contrast to other Golgi glycotransferases, it contains three transmembrane domains. This family also includes uncharacterized fungal proteins with similarity to PGAP4. |
| 409191 | PGAP4 | 7.17e-24 | 30 | 375 | 25 | 372 | Post-GPI attachment to proteins factor 4. Post-GPI attachment to proteins factor 4 (PGAP4), also known as post-GPI attachment to proteins GalNAc transferase 4 or transmembrane protein 246 (TMEM246), has been shown to be a Golgi-resident GPI-GalNAc transferase. Many eukaryotic proteins are anchored to the cell surface through glycolipid glycosylphosphatidylinositol (GPI). GPIs have a conserved core but exhibit diverse N-acetylgalactosamine (GalNAc) modifications. PGAP4 knockout cells lose GPI-GalNAc structures. PGAP4 is most likely involved in the initial steps of GPI-GalNAc biosynthesis. In contrast to other Golgi glycotransferases (GTs), it contains three transmembrane domains. Structural modeling suggests that PGAP4 adopts a GT-A fold split by an insertion of tandem transmembrane domains. |
| 398374 | Glyco_transf_54 | 4.00e-13 | 68 | 228 | 24 | 186 | N-Acetylglucosaminyltransferase-IV (GnT-IV) conserved region. The complex-type of oligosaccharides are synthesized through elongation by glycosyltransferases after trimming of the precursor oligosaccharides transferred to proteins in the endoplasmic reticulum. N-Acetylglucosaminyltransferases (GnTs) take part in the formation of branches in the biosynthesis of complex-type sugar chains. In vertebrates, six GnTs, designated as GnT-I to -VI, which catalyze the transfer of GlcNAc to the core mannose residues of Asn-linked sugar chains, have been identified. GnT-IV (EC:2.4.1.145) catalyzes the transfer of GlcNAc from UDP-GlcNAc to the GlcNAc1-2Man1-3 arm of core oligosaccharide [Gn2(22)core oligosaccharide] and forms GlcNAc1-4(GlcNAc1-2)Man1-3 structure on the core oligosaccharide (Gn3(2,4,2)core oligosaccharide). In some members the conserved region occupies all but the very for N-terminal, where there is a signal sequence on all members. For other members the conserved region does not occupy the entire protein but is still to the N-terminus of the protein. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.49e-312 | 1 | 424 | 1 | 424 | |
| 1.29e-182 | 4 | 416 | 6 | 417 | |
| 3.08e-167 | 1 | 415 | 1 | 419 | |
| 2.18e-101 | 14 | 409 | 36 | 433 | |
| 1.22e-99 | 26 | 411 | 41 | 436 |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.28e-07 | 69 | 202 | 133 | 276 | Alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase B OS=Danio rerio OX=7955 GN=mgat4bQ9UQ53 PE=2 SV=1 |
|
| 6.90e-07 | 81 | 202 | 139 | 270 | Alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase A OS=Xenopus laevis OX=8355 GN=mgat4a PE=2 SV=1 |
|
| 1.23e-06 | 81 | 197 | 146 | 272 | Alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase B OS=Mus musculus OX=10090 GN=Mgat4b PE=2 SV=1 |
|
| 2.12e-06 | 79 | 203 | 137 | 271 | Alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase A OS=Bos taurus OX=9913 GN=MGAT4A PE=1 SV=1 |
|
| 3.72e-06 | 81 | 202 | 139 | 270 | Alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase A OS=Xenopus tropicalis OX=8364 GN=mgat4a PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
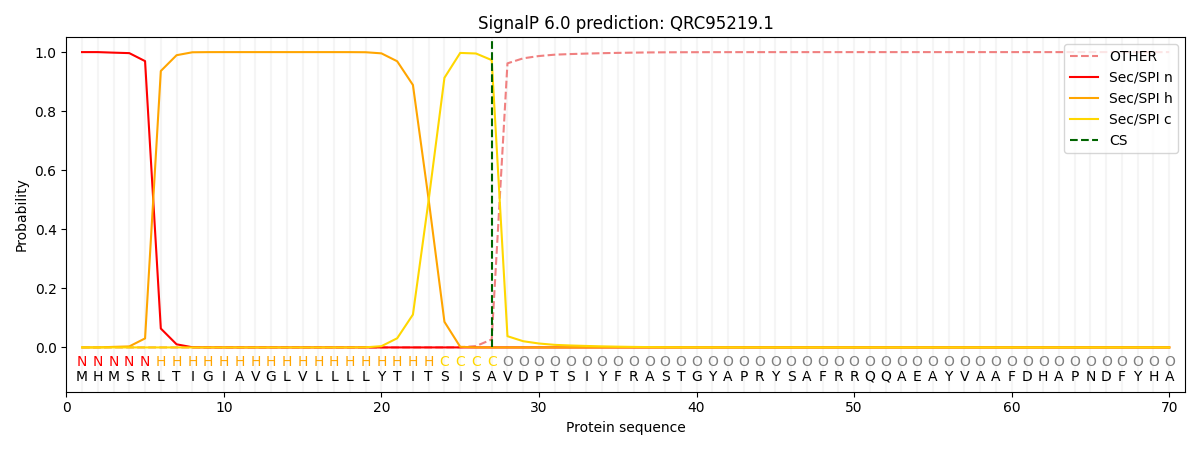
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000338 | 0.999623 | CS pos: 27-28. Pr: 0.9730 |

