You are browsing environment: FUNGIDB
CAZyme Information: PWY79584.1
You are here: Home > Sequence: PWY79584.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
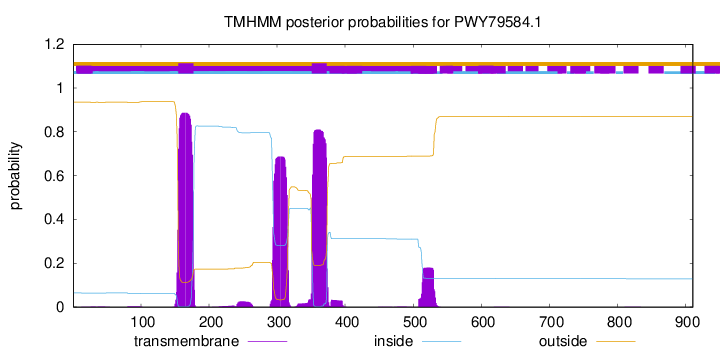
TMHMM annotations
Basic Information help
| Species | Aspergillus heteromorphus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus heteromorphus | |||||||||||
| CAZyme ID | PWY79584.1 | |||||||||||
| CAZy Family | GT76 | |||||||||||
| CAZyme Description | alkaline phosphatase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.96:2 |
|---|
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 119363 | GH18_CTS3_chitinase | 1.22e-138 | 12 | 266 | 1 | 256 | GH18 domain of CTS3 (chitinase 3), an uncharacterized protein from the human fungal pathogen Coccidioides posadasii. CTS3 has a chitinase-like glycosyl hydrolase family 18 (GH18) domain; and has homologs in bacteria as well as fungi. |
| 401396 | PhoD | 1.46e-47 | 478 | 808 | 1 | 342 | PhoD-like phosphatase. |
| 277335 | MPP_PhoD | 4.21e-34 | 477 | 765 | 1 | 241 | Bacillus subtilis PhoD and related proteins, metallophosphatase domain. PhoD (also known as alkaline phosphatase D/APaseD in Bacillus subtilis) is a secreted phosphodiesterase encoded by phoD of the Pho regulon in Bacillus subtilis. PhoD homologs are found in prokaryotes, eukaryotes, and archaea. PhoD contains a twin arginine (RR) motif and is transported by the Tat (Twin-arginine translocation) translocation pathway machinery (TatAyCy). This family also includes the Fusarium oxysporum Fso1 protein. PhoD belongs to the metallophosphatase (MPP) superfamily. MPPs are functionally diverse, but all share a conserved domain with an active site consisting of two metal ions (usually manganese, iron, or zinc) coordinated with octahedral geometry by a cage of histidine, aspartate, and asparagine residues. The MPP superfamily includes: Mre11/SbcD-like exonucleases, Dbr1-like RNA lariat debranching enzymes, YfcE-like phosphodiesterases, purple acid phosphatases (PAPs), YbbF-like UDP-2,3-diacylglucosamine hydrolases, and acid sphingomyelinases (ASMases). The conserved domain is a double beta-sheet sandwich with a di-metal active site made up of residues located at the C-terminal side of the sheets. This domain is thought to allow for productive metal coordination. |
| 226070 | PhoD | 1.01e-18 | 442 | 770 | 106 | 451 | Phosphodiesterase/alkaline phosphatase D [Inorganic ion transport and metabolism]. |
| 119349 | GH18_chitinase-like | 2.31e-16 | 13 | 196 | 1 | 177 | The GH18 (glycosyl hydrolase, family 18) type II chitinases hydrolyze chitin, an abundant polymer of beta-1,4-linked N-acetylglucosamine (GlcNAc) which is a major component of the cell wall of fungi and the exoskeleton of arthropods. Chitinases have been identified in viruses, bacteria, fungi, protozoan parasites, insects, and plants. The structure of the GH18 domain is an eight-stranded beta/alpha barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. The GH18 family includes chitotriosidase, chitobiase, hevamine, zymocin-alpha, narbonin, SI-CLP (stabilin-1 interacting chitinase-like protein), IDGF (imaginal disc growth factor), CFLE (cortical fragment-lytic enzyme) spore hydrolase, the type III and type V plant chitinases, the endo-beta-N-acetylglucosaminidases, and the chitolectins. The GH85 (glycosyl hydrolase, family 85) ENGases (endo-beta-N-acetylglucosaminidases) are closely related to the GH18 chitinases and are included in this alignment model. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 888 | 1 | 913 | |
| 0.0 | 1 | 888 | 1 | 913 | |
| 0.0 | 29 | 890 | 1 | 943 | |
| 0.0 | 1 | 884 | 36 | 910 | |
| 1.14e-174 | 1 | 293 | 1 | 293 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.91e-165 | 1 | 287 | 3 | 290 | Structure of chitinase, ChiC, from Aspergillus fumigatus. [Aspergillus fumigatus Af293],2Y8V_B Structure of chitinase, ChiC, from Aspergillus fumigatus. [Aspergillus fumigatus Af293],2Y8V_C Structure of chitinase, ChiC, from Aspergillus fumigatus. [Aspergillus fumigatus Af293],2Y8V_D Structure of chitinase, ChiC, from Aspergillus fumigatus. [Aspergillus fumigatus Af293] |
|
| 7.27e-75 | 12 | 286 | 4 | 280 | Chain X, ENDO-N-ACETYL-BETA-D-GLUCOSAMINIDASE [Trichoderma reesei] |
|
| 2.96e-12 | 69 | 270 | 57 | 269 | Crystal structure of a GH18 chitinase from Pseudoalteromonas aurantia [Pseudoalteromonas aurantia],6K7Z_B Crystal structure of a GH18 chitinase from Pseudoalteromonas aurantia [Pseudoalteromonas aurantia],6K7Z_C Crystal structure of a GH18 chitinase from Pseudoalteromonas aurantia [Pseudoalteromonas aurantia],6K7Z_D Crystal structure of a GH18 chitinase from Pseudoalteromonas aurantia [Pseudoalteromonas aurantia] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.47e-08 | 73 | 242 | 105 | 306 | Endochitinase 37 OS=Trichoderma harzianum OX=5544 GN=chit37 PE=1 SV=1 |
|
| 7.05e-08 | 73 | 197 | 57 | 194 | Endochitinase 4 (Fragment) OS=Metarhizium anisopliae OX=5530 GN=chi4 PE=3 SV=1 |
|
| 1.10e-07 | 73 | 269 | 108 | 343 | Endochitinase 4 OS=Metarhizium robertsii (strain ARSEF 23 / ATCC MYA-3075) OX=655844 GN=chi4 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
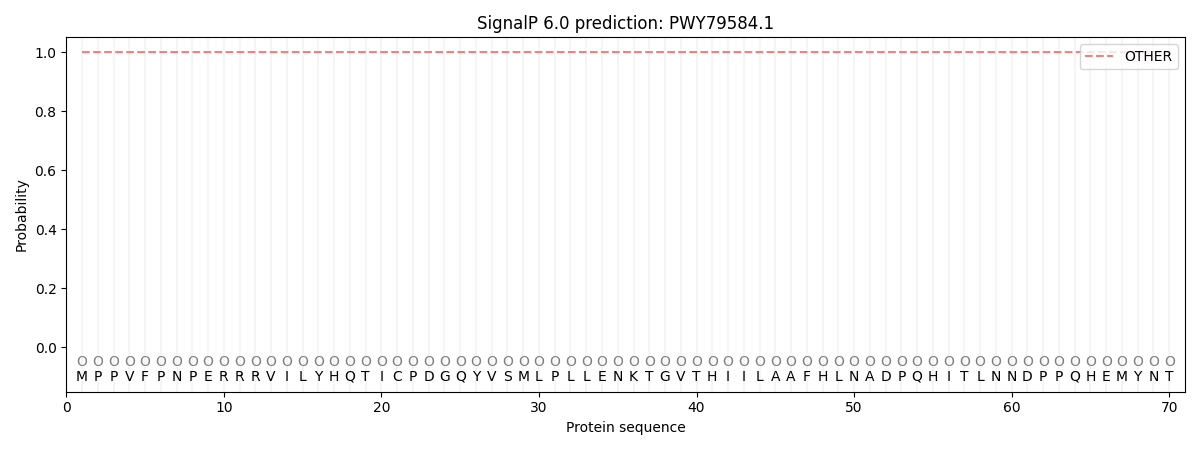
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000065 | 0.000000 |