You are browsing environment: FUNGIDB
CAZyme Information: PV10_05279-t45_4-p1
You are here: Home > Sequence: PV10_05279-t45_4-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Exophiala mesophila | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Exophiala; Exophiala mesophila | |||||||||||
| CAZyme ID | PV10_05279-t45_4-p1 | |||||||||||
| CAZy Family | GH47 | |||||||||||
| CAZyme Description | hypothetical protein, variant 3 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 40 | 222 | 3.4e-53 | 0.44692737430167595 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 259919 | CuRO_1_Abr2_like | 3.08e-65 | 26 | 142 | 1 | 117 | The first cupredoxin domain of a group of fungal Laccases similar to Abr2 from Aspergillus fumigatus. Abr2 is involved in conidial pigment biosynthesis in Aspergillus fumigatus. Laccase is a blue multi-copper enzyme that catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. Laccase has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism in fungi and plants. Like other related multicopper oxidases (MCOs), laccase is composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259926 | CuRO_1_Diphenol_Ox | 4.87e-58 | 24 | 142 | 1 | 119 | The first cupredoxin domain of fungal laccase, diphenol oxidase. Diphenol oxidase belongs to the laccase family. It catalyzes the initial steps in melanin biosynthesis from diphenols. Melanin is one of the virulence factors of infectious fungi. In the pathogenesis of C. neoformans, melanin pigments have been shown to protect the fungal cells from oxidative and microbicidal activities of host defense systems. Laccase is a blue multicopper oxidase (MCO) which catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259923 | CuRO_1_MaLCC_like | 9.42e-52 | 24 | 142 | 4 | 122 | The first cupredoxin domain of the fungal laccases similar to Ma-LCC from Melanocarpus albomyces. The subfamily of fungal laccases includes Ma-LCC and similar proteins. Ma-LCC is a multicopper oxidase (MCO) from Melanocarpus albomyces. Its crystal structure contains all four coppers at the mono- and trinuclear copper centers. Laccase is a blue multi-copper enzyme that catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism in fungi and plants. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259869 | CuRO_1_LCC_like | 1.61e-47 | 24 | 142 | 1 | 120 | Cupredoxin domain 1 of laccase-like multicopper oxidases; including laccase, CueO, spore coat protein A, ascorbate oxidase and similar proteins. Laccase-like multicopper oxidases (MCOs) in this family contain three cupredoxin domains. They are able to couple oxidation of substrates with reduction of dioxygen to water. MCOs are capable of oxidizing a vast range of substrates, varying from aromatic to inorganic compounds such as metals. Although the members of this family have diverse functions, majority of them have three cupredoxin domain repeats. The copper ions are bound in several sites; Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. Also included in this family are cupredoxin domains 1, 3, and 5 of the 6-domain MCO ceruloplasmin and similar proteins. |
| 400195 | Cu-oxidase_3 | 2.20e-47 | 29 | 146 | 1 | 119 | Multicopper oxidase. This entry contains many divergent copper oxidase-like domains that are not recognized by the pfam00394 model. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.44e-213 | 21 | 617 | 17 | 579 | |
| 4.91e-204 | 1 | 597 | 1 | 562 | |
| 1.06e-198 | 3 | 586 | 5 | 552 | |
| 2.02e-196 | 16 | 611 | 12 | 583 | |
| 6.23e-191 | 19 | 586 | 27 | 565 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.41e-31 | 27 | 211 | 7 | 176 | Crystal structure of LacB from Trametes sp. AH28-2 [Trametes sp. AH28-2],3KW7_B Crystal structure of LacB from Trametes sp. AH28-2 [Trametes sp. AH28-2] |
|
| 1.43e-29 | 9 | 211 | 11 | 197 | Crystal structure of laccases from Pycnoporus sanguineus, izoform I [Trametes sanguinea] |
|
| 5.10e-29 | 27 | 211 | 7 | 175 | T2-depleted laccase from Coriolopsis caperata soaked with CuCl [Coriolopsis caperata],4JHV_A T2-depleted laccase from Coriolopsis caperata [Coriolopsis caperata] |
|
| 6.29e-29 | 9 | 211 | 11 | 197 | Crystal structure of laccases from Pycnoporus sanguineus, izoform II [Trametes coccinea],5NQ9_A Crystal structure of laccases from Pycnoporus sanguineus, izoform II, monoclinic [Trametes sanguinea],5NQ9_C Crystal structure of laccases from Pycnoporus sanguineus, izoform II, monoclinic [Trametes sanguinea] |
|
| 9.50e-29 | 27 | 211 | 7 | 176 | Type-2 Cu-depleted fungus laccase from Trametes hirsuta [Trametes hirsuta] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.82e-62 | 24 | 553 | 23 | 439 | Laccase PFICI_06862 OS=Pestalotiopsis fici (strain W106-1 / CGMCC3.15140) OX=1229662 GN=PFICI_06862 PE=3 SV=1 |
|
| 6.64e-54 | 10 | 465 | 8 | 364 | Laccase abr2 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=abr2 PE=1 SV=1 |
|
| 2.05e-50 | 16 | 594 | 17 | 515 | Laccase-1 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=yA PE=2 SV=3 |
|
| 5.11e-47 | 27 | 505 | 27 | 406 | Multicopper oxidase MCE OS=Talaromyces pinophilus OX=128442 GN=MCE PE=1 SV=1 |
|
| 2.27e-44 | 4 | 583 | 4 | 504 | Laccase 1 OS=Metarhizium majus (strain ARSEF 297) OX=1276143 GN=Mlac1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
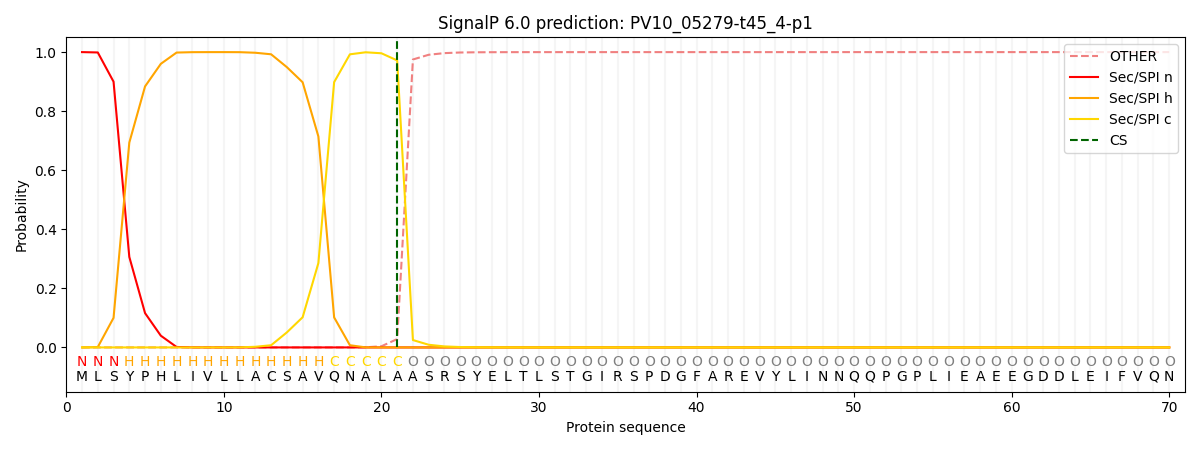
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000243 | 0.999720 | CS pos: 21-22. Pr: 0.9718 |
