You are browsing environment: FUNGIDB
CAZyme Information: PV06_11743-t43_1-p1
You are here: Home > Sequence: PV06_11743-t43_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
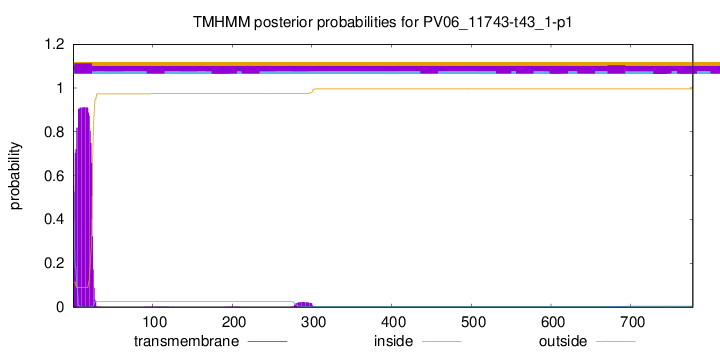
TMHMM annotations
Basic Information help
| Species | Exophiala oligosperma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Exophiala; Exophiala oligosperma | |||||||||||
| CAZyme ID | PV06_11743-t43_1-p1 | |||||||||||
| CAZy Family | GT71 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 344 | 518 | 2e-49 | 0.4441340782122905 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 259862 | CuRO_1_ceruloplasmin_like | 9.69e-65 | 31 | 208 | 1 | 176 | Cupredoxin domains 1, 3, and 5 of ceruloplasmin and similar proteins. This family includes the first, third, and fifth cupredoxin domains of ceruloplasmin and similar proteins including the first, third and fifth cupredoxin domains of unprocessed coagulation factors V and VIII. Ceruloplasmin (ferroxidase) is a multicopper oxidase essential for normal iron homeostasis. It functions in copper transport, amine oxidation and as an antioxidant preventing free radicals in serum. The protein has 6 cupredoxin domains and exhibits internal sequence homology that appears to have evolved from the triplication of a sequence unit composed of two tandem cupredoxin domains. Human Factor VIII facilitates blood clotting by acting as a cofactor for factor IXa. Factor VIII and IXa forms a complex in the presence of Ca+2 and phospholipids that converts factor X to the activated form Xa. |
| 259884 | CuRO_1_ceruloplasmin | 2.89e-58 | 31 | 206 | 1 | 180 | The first cupredoxin domain of Ceruloplasmin. Ceruloplasmin is a multicopper oxidase essential for normal iron homeostasis and copper transport in blood. It also functions in amine oxidation and as an antioxidant preventing free radicals in serum. The protein has 6 cupredoxin domains with six copper centers; three mononuclear sites in domain 2, 4 and 6 and three in the form of trinuclear clusters at the interface of domains 1 and 6. Ceruloplasmin exhibits internal sequence homology that appears to have evolved from the triplication of a sequence unit composed of two tandem cupredoxin domains. This model represents the first cupredoxin domain of ceruloplasmin. |
| 259919 | CuRO_1_Abr2_like | 7.45e-57 | 342 | 446 | 13 | 117 | The first cupredoxin domain of a group of fungal Laccases similar to Abr2 from Aspergillus fumigatus. Abr2 is involved in conidial pigment biosynthesis in Aspergillus fumigatus. Laccase is a blue multi-copper enzyme that catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. Laccase has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism in fungi and plants. Like other related multicopper oxidases (MCOs), laccase is composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259886 | CuRO_3_ceruloplasmin | 5.95e-55 | 28 | 215 | 1 | 193 | The third cupredoxin domain of Ceruloplasmin. Ceruloplasmin is a multicopper oxidase essential for normal iron homeostasis and copper transport in blood. It also functions in amine oxidation and as an antioxidant preventing free radicals in serum. The protein has 6 cupredoxin domains with six copper centers; three mononuclear sites in domain 2, 4 and 6 and three in the form of trinuclear clusters at the interface of domains 1 and 6. Ceruloplasmin exhibits internal sequence homology that appears to have evolved from the triplication of a sequence unit composed of two tandem cupredoxin domains. This model represents the third cupredoxin domain of ceruloplasmin. |
| 259891 | CuRO_1_Ceruloplasmin_like_1 | 1.24e-53 | 31 | 210 | 1 | 175 | cupredoxin domain of ceruloplasmin homologs. Uncharacterized subfamily of ceruloplasmin homologous proteins. Ceruloplasmin (ferroxidase) is a multicopper oxidase essential for normal iron homeostasis. Ceruloplasmin also functions in copper transport, amine oxidase and as an antioxidant preventing free radicals in serum. The protein has 6 cupredoxin domains and exhibits internal sequence homology that appears to have evolved from the triplication of a sequence unit composed of two tandem cupredoxin domains. This model represents the first domain of the triplicated units. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.26e-156 | 342 | 767 | 34 | 430 | |
| 1.76e-150 | 333 | 764 | 42 | 434 | |
| 1.13e-146 | 338 | 764 | 37 | 426 | |
| 6.19e-144 | 339 | 764 | 37 | 430 | |
| 1.35e-140 | 339 | 769 | 43 | 446 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.15e-51 | 31 | 340 | 707 | 997 | Rat ceruloplasmin trigonal form [Rattus norvegicus],5N4L_B Rat ceruloplasmin trigonal form [Rattus norvegicus] |
|
| 2.30e-51 | 31 | 340 | 726 | 1016 | Rat ceruloplasmin orthorhombic form [Rattus norvegicus] |
|
| 1.35e-48 | 31 | 340 | 713 | 1003 | X-Ray Crystal Structure Of Human Ceruloplasmin At 3.0 Angstroms [Homo sapiens] |
|
| 1.40e-48 | 31 | 340 | 732 | 1022 | Ceruloplasmin revisited: structural and functional roles of various metal cation binding sites [Homo sapiens],4EJX_A Structure of ceruloplasmin-myeloperoxidase complex [Homo sapiens] |
|
| 1.40e-48 | 31 | 340 | 732 | 1022 | Structure of human ceruloplasmin at 2.6 A resolution [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.50e-65 | 27 | 368 | 728 | 1066 | Hephaestin-like protein OS=Acropora millepora OX=45264 PE=1 SV=1 |
|
| 2.82e-60 | 26 | 334 | 21 | 325 | Ferroxidase HEPHL1 OS=Mus musculus OX=10090 GN=Hephl1 PE=2 SV=2 |
|
| 5.50e-59 | 26 | 379 | 21 | 364 | Ferroxidase HEPHL1 OS=Homo sapiens OX=9606 GN=HEPHL1 PE=1 SV=2 |
|
| 3.65e-56 | 19 | 334 | 14 | 326 | Hephaestin OS=Rattus norvegicus OX=10116 GN=Heph PE=1 SV=1 |
|
| 3.66e-56 | 27 | 334 | 22 | 326 | Hephaestin OS=Homo sapiens OX=9606 GN=HEPH PE=1 SV=3 |
SignalP and Lipop Annotations help
This protein is predicted as SP
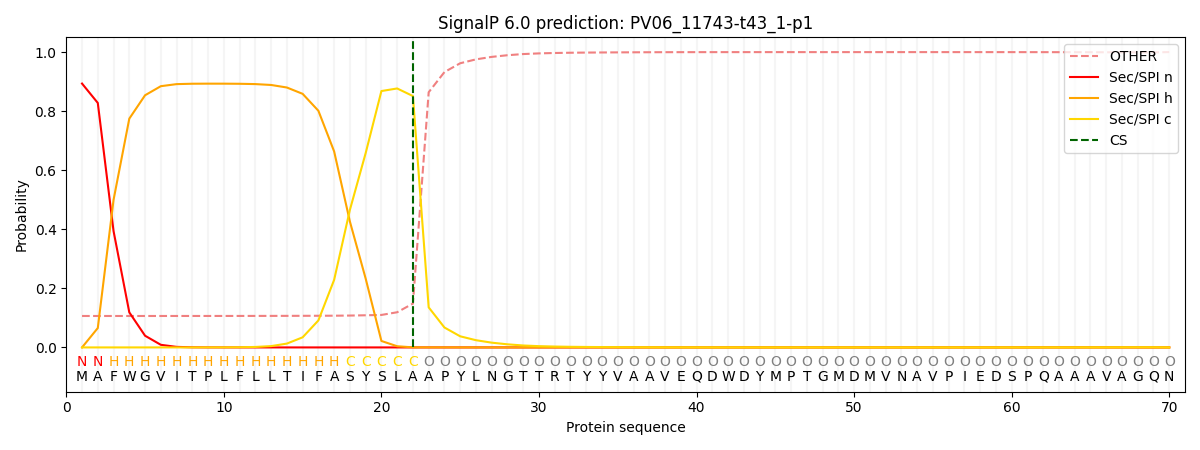
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.116265 | 0.883693 | CS pos: 22-23. Pr: 0.8512 |