You are browsing environment: FUNGIDB
CAZyme Information: PV06_10222-t43_1-p1
You are here: Home > Sequence: PV06_10222-t43_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Exophiala oligosperma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Exophiala; Exophiala oligosperma | |||||||||||
| CAZyme ID | PV06_10222-t43_1-p1 | |||||||||||
| CAZy Family | GT2|GT2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.49:3 | 3.2.1.22:2 | 3.2.1.49:2 | 3.2.1.22:2 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH27 | 127 | 387 | 1.8e-52 | 0.9737991266375546 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 269893 | GH27 | 1.04e-100 | 33 | 322 | 1 | 271 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| 166449 | PLN02808 | 1.87e-73 | 29 | 409 | 28 | 380 | alpha-galactosidase |
| 374582 | Melibiase_2 | 1.47e-71 | 33 | 322 | 2 | 284 | Alpha galactosidase A. |
| 178295 | PLN02692 | 9.32e-67 | 1 | 386 | 20 | 381 | alpha-galactosidase |
| 177874 | PLN02229 | 2.30e-58 | 29 | 386 | 59 | 390 | alpha-galactosidase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 4.16e-199 | 15 | 551 | 13 | 542 | |
| 7.50e-193 | 17 | 550 | 20 | 544 | |
| 1.93e-192 | 19 | 550 | 18 | 529 | |
| 1.22e-189 | 17 | 550 | 12 | 536 | |
| 2.06e-187 | 6 | 550 | 8 | 532 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.93e-59 | 29 | 403 | 5 | 351 | Chain A, alpha-galactosidase [Oryza sativa] |
|
| 4.10e-59 | 33 | 356 | 100 | 420 | Crystal structure of a putative alpha-galactosidase/melibiase (BF4189) from Bacteroides fragilis NCTC 9343 at 2.00 A resolution [Bacteroides fragilis NCTC 9343],4OGZ_B Crystal structure of a putative alpha-galactosidase/melibiase (BF4189) from Bacteroides fragilis NCTC 9343 at 2.00 A resolution [Bacteroides fragilis NCTC 9343] |
|
| 4.41e-57 | 29 | 358 | 5 | 327 | Chain A, Alpha-galactosidase A [Homo sapiens],3LX9_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXA_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXA_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXB_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXB_B Chain B, Alpha-galactosidase A [Homo sapiens],3LXC_A Chain A, Alpha-galactosidase A [Homo sapiens],3LXC_B Chain B, Alpha-galactosidase A [Homo sapiens] |
|
| 7.60e-56 | 29 | 358 | 5 | 327 | Structure of human alpha-galactosidase [Homo sapiens],1R46_B Structure of human alpha-galactosidase [Homo sapiens],1R47_A Structure of human alpha-galactosidase [Homo sapiens],1R47_B Structure of human alpha-galactosidase [Homo sapiens],3GXN_A Crystal structure of apo alpha-galactosidase A at pH 4.5 [Homo sapiens],3GXN_B Crystal structure of apo alpha-galactosidase A at pH 4.5 [Homo sapiens],3GXP_A Crystal structure of acid-alpha-galactosidase A complexed with galactose at pH 4.5 [Homo sapiens],3GXP_B Crystal structure of acid-alpha-galactosidase A complexed with galactose at pH 4.5 [Homo sapiens],3GXT_A Crystal structure of alpha-galactosidase A at pH 4.5 complexed with 1-deoxygalactonijirimycin [Homo sapiens],3GXT_B Crystal structure of alpha-galactosidase A at pH 4.5 complexed with 1-deoxygalactonijirimycin [Homo sapiens],3HG2_A Human alpha-galactosidase catalytic mechanism 1. Empty active site [Homo sapiens],3HG2_B Human alpha-galactosidase catalytic mechanism 1. Empty active site [Homo sapiens],3HG4_A Human alpha-galactosidase catalytic mechanism 3. Covalent intermediate [Homo sapiens],3HG4_B Human alpha-galactosidase catalytic mechanism 3. Covalent intermediate [Homo sapiens],3HG5_A Human alpha-galactosidase catalytic mechanism 4. Product bound [Homo sapiens],3HG5_B Human alpha-galactosidase catalytic mechanism 4. Product bound [Homo sapiens],3S5Y_A Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Y_B Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Z_A Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],3S5Z_B Pharmacological Chaperoning in Human alpha-Galactosidase [Homo sapiens],4NXS_A Crystal structure of human alpha-galactosidase A in complex with 1-deoxygalactonojirimycin-pFPhT [Homo sapiens],4NXS_B Crystal structure of human alpha-galactosidase A in complex with 1-deoxygalactonojirimycin-pFPhT [Homo sapiens],6IBK_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfamidate ME763 [Homo sapiens],6IBK_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfamidate ME763 [Homo sapiens],6IBM_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfate ME776 [Homo sapiens],6IBM_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclosulfate ME776 [Homo sapiens],6IBR_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol epoxide LWA481 [Homo sapiens],6IBR_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol epoxide LWA481 [Homo sapiens],6IBT_A Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol aziridine ME737 [Homo sapiens],6IBT_B Crystal structure of human alpha-galactosidase A in complex with alpha-galactose configured cyclophellitol aziridine ME737 [Homo sapiens] |
|
| 1.26e-55 | 29 | 390 | 5 | 360 | The Structure of alpha-N-Acetylgalactosaminidase [Gallus gallus],1KTC_A The Structure of alpha-N-Acetylgalactosaminidase [Gallus gallus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.69e-195 | 19 | 552 | 18 | 531 | Probable alpha-galactosidase A OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=aglA PE=3 SV=1 |
|
| 1.33e-193 | 17 | 550 | 20 | 544 | Alpha-galactosidase A OS=Aspergillus niger OX=5061 GN=aglA PE=1 SV=1 |
|
| 3.43e-193 | 19 | 550 | 18 | 529 | Probable alpha-galactosidase A OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=aglA PE=3 SV=1 |
|
| 5.04e-191 | 7 | 550 | 3 | 523 | Probable alpha-galactosidase A OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=aglA PE=3 SV=1 |
|
| 2.17e-190 | 17 | 550 | 12 | 536 | Probable alpha-galactosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=aglA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
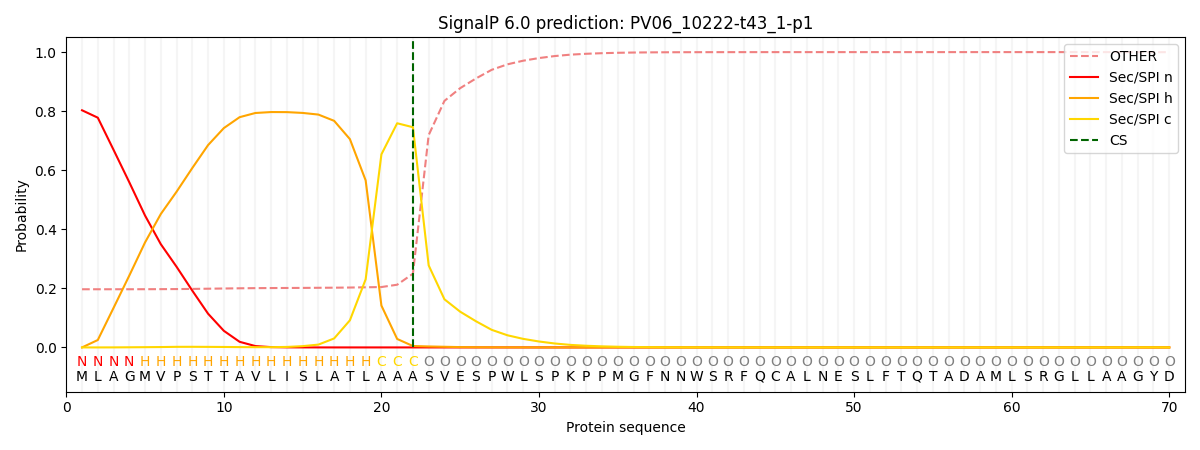
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.207666 | 0.792298 | CS pos: 22-23. Pr: 0.7456 |
