You are browsing environment: FUNGIDB
CAZyme Information: PSURA_86067T0-p1
You are here: Home > Sequence: PSURA_86067T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phytophthora ramorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora ramorum | |||||||||||
| CAZyme ID | PSURA_86067T0-p1 | |||||||||||
| CAZy Family | GT71 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 2 | 217 | 2.3e-25 | 0.8588235294117647 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173823 | plant_peroxidase_like | 7.54e-52 | 1 | 215 | 28 | 254 | Heme-dependent peroxidases similar to plant peroxidases. Along with animal peroxidases, these enzymes belong to a group of peroxidases containing a heme prosthetic group (ferriprotoporphyrin IX), which catalyzes a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. The plant peroxidase-like superfamily is found in all three kingdoms of life and carries out a variety of biosynthetic and degradative functions. Several sub-families can be identified. Class I includes intracellular peroxidases present in fungi, plants, archaea and bacteria, called catalase-peroxidases, that can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. Catalase-peroxidases are typically comprised of two homologous domains that probably arose via a single gene duplication event. Class II includes ligninase and other extracellular fungal peroxidases, while class III is comprised of classic extracellular plant peroxidases, like horseradish peroxidase. |
| 173825 | ascorbate_peroxidase | 9.56e-36 | 1 | 213 | 40 | 244 | Ascorbate peroxidases and cytochrome C peroxidases. Ascorbate peroxidases are a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Along with related catalase-peroxidases, ascorbate peroxidases belong to class I of the plant superfamily. Ascorbate peroxidases are found in the chloroplasts and/or cytosol of algae and plants, where they have been shown to control the concentration of lethal hydrogen peroxide molecules. The yeast cytochrome c peroxidase is a divergent member of the family; it forms a complex with cytochrome c to catalyze the reduction of hydrogen peroxide to water. |
| 223453 | KatG | 3.95e-34 | 2 | 221 | 476 | 728 | Catalase (peroxidase I) [Inorganic ion transport and metabolism]. |
| 173828 | catalase_peroxidase_2 | 4.53e-34 | 1 | 218 | 40 | 296 | C-terminal non-catalytic domain of catalase-peroxidases. This is a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Catalase-peroxidases can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. These enzymes are found in many archaeal and bacterial organisms where they neutralize potentially lethal hydrogen peroxide molecules generated during photosynthesis or stationary phase. Along with related intracellular fungal and plant peroxidases, catalase-peroxidases belong to plant peroxidase superfamily. Unlike the eukaryotic enzymes, they are typically comprised of two homologous domains that probably arose via a single gene duplication event. The heme binding motif is present only in the N-terminal domain; the function of the C-terminal domain is not clear. |
| 237891 | PRK15061 | 5.43e-30 | 2 | 220 | 466 | 723 | catalase/peroxidase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 6.66e-140 | 1 | 225 | 463 | 687 | |
| 4.10e-127 | 1 | 225 | 463 | 687 | |
| 1.64e-126 | 1 | 225 | 463 | 687 | |
| 7.02e-28 | 2 | 214 | 99 | 322 | |
| 1.17e-26 | 6 | 215 | 57 | 275 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.16e-23 | 12 | 214 | 47 | 254 | Structure of Leishmania major peroxidase D211N mutant [Leishmania major],5AMM_B Structure of Leishmania major peroxidase D211N mutant [Leishmania major] |
|
| 3.21e-23 | 12 | 214 | 48 | 255 | The Crystal Structure of Leishmania major Peroxidase mutant C197T [Leishmania major strain Friedlin],3RIW_B The Crystal Structure of Leishmania major Peroxidase mutant C197T [Leishmania major strain Friedlin] |
|
| 5.91e-23 | 12 | 214 | 47 | 254 | Crystal Structure of the Leishmania Major Peroxidase-Cytochrome C Complex [Leishmania major] |
|
| 6.23e-23 | 12 | 214 | 48 | 255 | The Crystal Structure of Leishmania major Peroxidase [Leishmania major strain Friedlin],3RIV_B The Crystal Structure of Leishmania major Peroxidase [Leishmania major strain Friedlin] |
|
| 8.54e-23 | 12 | 214 | 47 | 254 | Structure of Leishmania major peroxidase D211R mutant (high res) [Leishmania major],5ALA_A Structure of Leishmania major peroxidase D211R mutant (low res) [Leishmania major],5ALA_B Structure of Leishmania major peroxidase D211R mutant (low res) [Leishmania major] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.12e-31 | 1 | 215 | 62 | 282 | Putative heme-binding peroxidase OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=CCP2 PE=3 SV=2 |
|
| 7.85e-27 | 6 | 215 | 57 | 275 | Putative heme-binding peroxidase OS=Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) OX=229533 GN=FGRRES_10606 PE=3 SV=1 |
|
| 3.76e-25 | 2 | 221 | 484 | 739 | Catalase-peroxidase OS=Leptospira biflexa serovar Patoc (strain Patoc 1 / ATCC 23582 / Paris) OX=456481 GN=katG PE=3 SV=1 |
|
| 3.76e-25 | 2 | 221 | 484 | 739 | Catalase-peroxidase OS=Leptospira biflexa serovar Patoc (strain Patoc 1 / Ames) OX=355278 GN=katG PE=3 SV=1 |
|
| 9.42e-25 | 2 | 221 | 477 | 735 | Catalase-peroxidase OS=Caulobacter sp. (strain K31) OX=366602 GN=katG PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
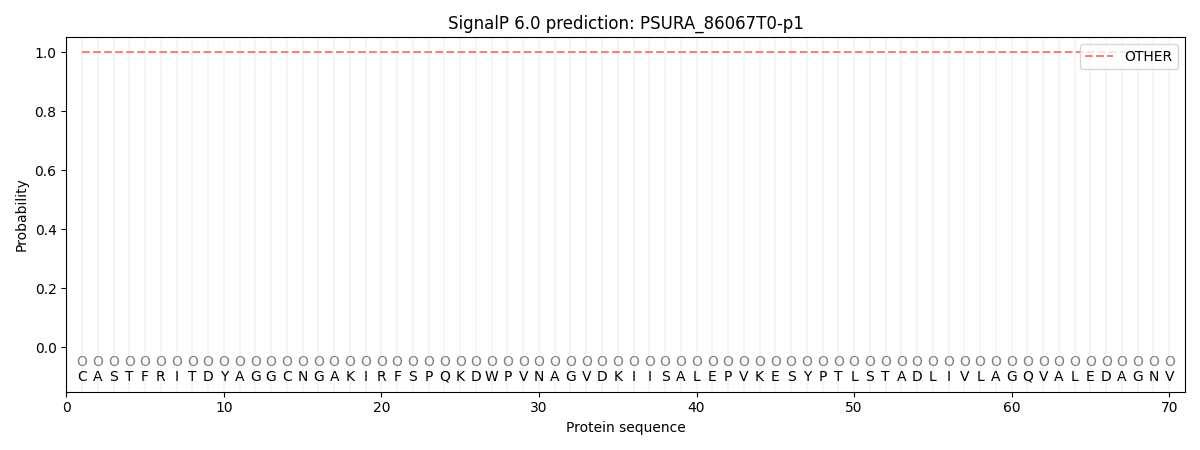
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000055 | 0.000001 |
