You are browsing environment: FUNGIDB
CAZyme Information: PSURA_74794T0-p1
You are here: Home > Sequence: PSURA_74794T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
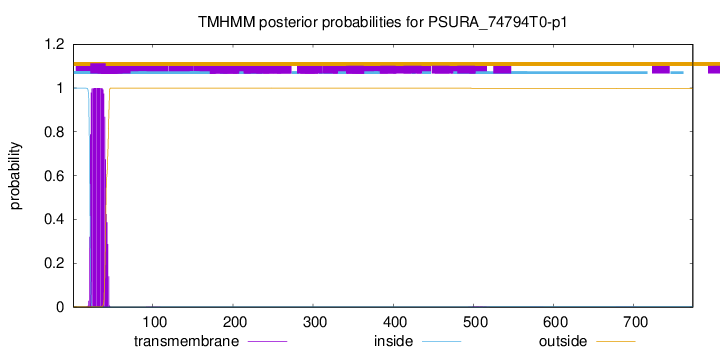
TMHMM annotations
Basic Information help
| Species | Phytophthora ramorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora ramorum | |||||||||||
| CAZyme ID | PSURA_74794T0-p1 | |||||||||||
| CAZy Family | GH123 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT71 | 91 | 341 | 4.5e-41 | 0.928030303030303 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 402574 | Mannosyl_trans3 | 2.36e-25 | 91 | 330 | 2 | 242 | Mannosyltransferase putative. This family is conserved in fungi. Several members are annotated as being alpha-1,3-mannosyltransferase but this could not be confirmed. |
| 212492 | retinol-DH_like_SDR_c_like | 2.09e-20 | 497 | 761 | 2 | 269 | retinol dehydrogenase (retinol-DH), Light dependent Protochlorophyllide (Pchlide) OxidoReductase (LPOR) and related proteins, classical (c) SDRs. Classical SDR subgroup containing retinol-DHs, LPORs, and related proteins. Retinol is processed by a medium chain alcohol dehydrogenase followed by retinol-DHs. Pchlide reductases act in chlorophyll biosynthesis. There are distinct enzymes that catalyze Pchlide reduction in light or dark conditions. Light-dependent reduction is via an NADP-dependent SDR, LPOR. Proteins in this subfamily share the glycine-rich NAD-binding motif of the classical SDRs, have a partial match to the canonical active site tetrad, but lack the typical active site Ser. This subgroup includes the human proteins: retinol dehydrogenase -12, -13 ,and -14, dehydrogenase/reductase SDR family member (DHRS)-12 , -13 and -X (a DHRS on chromosome X), and WWOX (WW domain-containing oxidoreductase), as well as a Neurospora crassa SDR encoded by the blue light inducible bli-4 gene. SDRs are a functionally diverse family of oxidoreductases that have a single domain with a structurally conserved Rossmann fold (alpha/beta folding pattern with a central beta-sheet), an NAD(P)(H)-binding region, and a structurally diverse C-terminal region. Classical SDRs are typically about 250 residues long, while extended SDRs are approximately 350 residues. Sequence identity between different SDR enzymes are typically in the 15-30% range, but the enzymes share the Rossmann fold NAD-binding motif and characteristic NAD-binding and catalytic sequence patterns. These enzymes catalyze a wide range of activities including the metabolism of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, and act in redox sensing. Classical SDRs have an TGXXX[AG]XG cofactor binding motif and a YXXXK active site motif, with the Tyr residue of the active site motif serving as a critical catalytic residue (Tyr-151, human 15-hydroxyprostaglandin dehydrogenase (15-PGDH) numbering). In addition to the Tyr and Lys, there is often an upstream Ser (Ser-138, 15-PGDH numbering) and/or an Asn (Asn-107, 15-PGDH numbering) contributing to the active site; while substrate binding is in the C-terminal region, which determines specificity. The standard reaction mechanism is a 4-pro-S hydride transfer and proton relay involving the conserved Tyr and Lys, a water molecule stabilized by Asn, and nicotinamide. Extended SDRs have additional elements in the C-terminal region, and typically have a TGXXGXXG cofactor binding motif. Complex (multidomain) SDRs such as ketoreductase domains of fatty acid synthase have a GGXGXXG NAD(P)-binding motif and an altered active site motif (YXXXN). Fungal type ketoacyl reductases have a TGXXXGX(1-2)G NAD(P)-binding motif. Some atypical SDRs have lost catalytic activity and/or have an unusual NAD(P)-binding motif and missing or unusual active site residues. Reactions catalyzed within the SDR family include isomerization, decarboxylation, epimerization, C=N bond reduction, dehydratase activity, dehalogenation, Enoyl-CoA reduction, and carbonyl-alcohol oxidoreduction. |
| 235627 | PRK05854 | 3.66e-14 | 497 | 647 | 15 | 170 | SDR family oxidoreductase. |
| 187670 | LPOR_like_SDR_c_like | 7.19e-12 | 497 | 732 | 2 | 268 | light-dependent protochlorophyllide reductase (LPOR)-like, classical (c)-like SDRs. Classical SDR-like subgroup containing LPOR and related proteins. Protochlorophyllide (Pchlide) reductases act in chlorophyll biosynthesis. There are distinct enzymes that catalyze Pchlide reduction in light or dark conditions. Light-dependent reduction is via an NADP-dependent SDR, LPOR. Proteins in this subfamily share the glycine-rich NAD-binding motif of the classical SDRs, have a partial match to the canonical active site tetrad, but lack the typical active site Ser. SDRs are a functionally diverse family of oxidoreductases that have a single domain with a structurally conserved Rossmann fold (alpha/beta folding pattern with a central beta-sheet), an NAD(P)(H)-binding region, and a structurally diverse C-terminal region. Classical SDRs are typically about 250 residues long, while extended SDRs are approximately 350 residues. Sequence identity between different SDR enzymes are typically in the 15-30% range, but the enzymes share the Rossmann fold NAD-binding motif and characteristic NAD-binding and catalytic sequence patterns. These enzymes catalyze a wide range of activities including the metabolism of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, and act in redox sensing. Classical SDRs have an TGXXX[AG]XG cofactor binding motif and a YXXXK active site motif, with the Tyr residue of the active site motif serving as a critical catalytic residue (Tyr-151, human 15-hydroxyprostaglandin dehydrogenase (15-PGDH) numbering). In addition to the Tyr and Lys, there is often an upstream Ser (Ser-138, 15-PGDH numbering) and/or an Asn (Asn-107, 15-PGDH numbering) contributing to the active site; while substrate binding is in the C-terminal region, which determines specificity. The standard reaction mechanism is a 4-pro-S hydride transfer and proton relay involving the conserved Tyr and Lys, a water molecule stabilized by Asn, and nicotinamide. Extended SDRs have additional elements in the C-terminal region, and typically have a TGXXGXXG cofactor binding motif. Complex (multidomain) SDRs such as ketoreductase domains of fatty acid synthase have a GGXGXXG NAD(P)-binding motif and an altered active site motif (YXXXN). Fungal type ketoacyl reductases have a TGXXXGX(1-2)G NAD(P)-binding motif. Some atypical SDRs have lost catalytic activity and/or have an unusual NAD(P)-binding motif and missing or unusual active site residues. Reactions catalyzed within the SDR family include isomerization, decarboxylation, epimerization, C=N bond reduction, dehydratase activity, dehalogenation, Enoyl-CoA reduction, and carbonyl-alcohol oxidoreduction. |
| 212493 | HetN_like_SDR_c | 6.60e-10 | 497 | 694 | 1 | 183 | HetN oxidoreductase-like, classical (c) SDR. This subgroup includes Anabaena sp. strain PCC 7120 HetN, a putative oxidoreductase involved in heterocyst differentiation, and related proteins. SDRs are a functionally diverse family of oxidoreductases that have a single domain with a structurally conserved Rossmann fold (alpha/beta folding pattern with a central beta-sheet), an NAD(P)(H)-binding region, and a structurally diverse C-terminal region. Classical SDRs are typically about 250 residues long, while extended SDRs are approximately 350 residues. Sequence identity between different SDR enzymes are typically in the 15-30% range, but the enzymes share the Rossmann fold NAD-binding motif and characteristic NAD-binding and catalytic sequence patterns. These enzymes catalyze a wide range of activities including the metabolism of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, and act in redox sensing. Classical SDRs have an TGXXX[AG]XG cofactor binding motif and a YXXXK active site motif, with the Tyr residue of the active site motif serving as a critical catalytic residue (Tyr-151, human 15-hydroxyprostaglandin dehydrogenase (15-PGDH) numbering). In addition to the Tyr and Lys, there is often an upstream Ser (Ser-138, 15-PGDH numbering) and/or an Asn (Asn-107, 15-PGDH numbering) contributing to the active site; while substrate binding is in the C-terminal region, which determines specificity. The standard reaction mechanism is a 4-pro-S hydride transfer and proton relay involving the conserved Tyr and Lys, a water molecule stabilized by Asn, and nicotinamide. Extended SDRs have additional elements in the C-terminal region, and typically have a TGXXGXXG cofactor binding motif. Complex (multidomain) SDRs such as ketoreductase domains of fatty acid synthase have a GGXGXXG NAD(P)-binding motif and an altered active site motif (YXXXN). Fungal type ketoacyl reductases have a TGXXXGX(1-2)G NAD(P)-binding motif. Some atypical SDRs have lost catalytic activity and/or have an unusual NAD(P)-binding motif and missing or unusual active site residues. Reactions catalyzed within the SDR family include isomerization, decarboxylation, epimerization, C=N bond reduction, dehydratase activity, dehalogenation, Enoyl-CoA reduction, and carbonyl-alcohol oxidoreduction. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.74e-107 | 39 | 470 | 58 | 520 | |
| 5.09e-102 | 19 | 465 | 8 | 430 | |
| 3.89e-80 | 91 | 377 | 66 | 358 | |
| 1.04e-62 | 69 | 451 | 61 | 454 | |
| 2.51e-55 | 91 | 438 | 65 | 437 |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.88e-18 | 499 | 769 | 22 | 328 | Oxidoreductase AN1596 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=AN1596 PE=2 SV=1 |
|
| 1.54e-16 | 499 | 691 | 23 | 220 | NmrA-like family domain-containing oxidoreductase notO OS=Aspergillus sp. (strain MF297-2) OX=877550 GN=notO PE=1 SV=1 |
|
| 3.46e-13 | 499 | 696 | 23 | 221 | Oxidoreductase andH OS=Emericella variicolor OX=1549217 GN=andH PE=1 SV=1 |
|
| 6.41e-10 | 499 | 766 | 46 | 321 | Dehydrogenase/reductase SDR family member on chromosome X homolog OS=Mus musculus OX=10090 GN=Dhrsx PE=1 SV=2 |
|
| 1.95e-08 | 499 | 766 | 46 | 321 | Dehydrogenase/reductase SDR family member on chromosome X OS=Homo sapiens OX=9606 GN=DHRSX PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
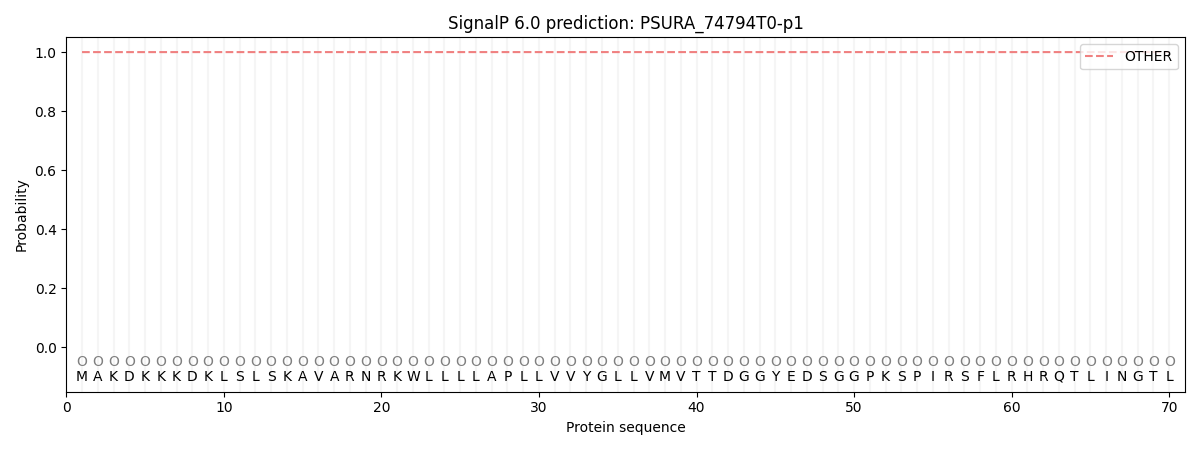
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000021 | 0.000002 |