You are browsing environment: FUNGIDB
CAZyme Information: PPTG_22560-t26_1-p1
You are here: Home > Sequence: PPTG_22560-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phytophthora parasitica | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora parasitica | |||||||||||
| CAZyme ID | PPTG_22560-t26_1-p1 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM13 | 252 | 348 | 9.8e-17 | 0.5319148936170213 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 227625 | Scw11 | 6.39e-29 | 54 | 243 | 90 | 277 | Exo-beta-1,3-glucanase, GH17 family [Carbohydrate transport and metabolism]. |
| 395527 | Ricin_B_lectin | 1.51e-19 | 242 | 343 | 29 | 126 | Ricin-type beta-trefoil lectin domain. |
| 238092 | RICIN | 1.15e-13 | 242 | 343 | 27 | 122 | Ricin-type beta-trefoil; Carbohydrate-binding domain formed from presumed gene triplication. The domain is found in a variety of molecules serving diverse functions such as enzymatic activity, inhibitory toxicity and signal transduction. Highly specific ligand binding occurs on exposed surfaces of the compact domain sturcture. |
| 395527 | Ricin_B_lectin | 9.89e-13 | 265 | 345 | 4 | 84 | Ricin-type beta-trefoil lectin domain. |
| 238092 | RICIN | 2.50e-12 | 270 | 347 | 8 | 84 | Ricin-type beta-trefoil; Carbohydrate-binding domain formed from presumed gene triplication. The domain is found in a variety of molecules serving diverse functions such as enzymatic activity, inhibitory toxicity and signal transduction. Highly specific ligand binding occurs on exposed surfaces of the compact domain sturcture. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.07e-204 | 1 | 351 | 1 | 443 | |
| 3.07e-204 | 1 | 351 | 1 | 443 | |
| 3.07e-204 | 1 | 351 | 1 | 443 | |
| 3.07e-204 | 1 | 351 | 1 | 443 | |
| 3.07e-204 | 1 | 351 | 1 | 443 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.05e-17 | 51 | 241 | 78 | 260 | Crystal structure of glycoside hydrolase family 17 beta-1,3-glucanosyltransferase from Rhizomucor miehei [Rhizomucor miehei CAU432] |
|
| 1.32e-16 | 51 | 241 | 78 | 260 | Active-site mutant of Rhizomucor miehei beta-1,3-glucanosyltransferase in complex with laminaribiose [Rhizomucor miehei CAU432],4WTS_A Active-site mutant of Rhizomucor miehei beta-1,3-glucanosyltransferase in complex with laminaritriose [Rhizomucor miehei CAU432] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 9.47e-14 | 51 | 243 | 431 | 640 | Probable glucan endo-1,3-beta-glucosidase btgC OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=btgC PE=3 SV=1 |
|
| 1.36e-13 | 58 | 225 | 71 | 249 | Glucan 1,3-beta-glucosidase OS=Candida albicans OX=5476 GN=BGL2 PE=3 SV=1 |
|
| 1.69e-13 | 51 | 243 | 431 | 640 | Probable glucan endo-1,3-beta-glucosidase btgC OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=btgC PE=3 SV=1 |
|
| 1.69e-13 | 51 | 243 | 431 | 640 | Probable glucan endo-1,3-beta-glucosidase btgC OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=btgC PE=3 SV=1 |
|
| 3.38e-13 | 53 | 230 | 68 | 247 | Glucan 1,3-beta-glucosidase ARB_02797 OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_02797 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
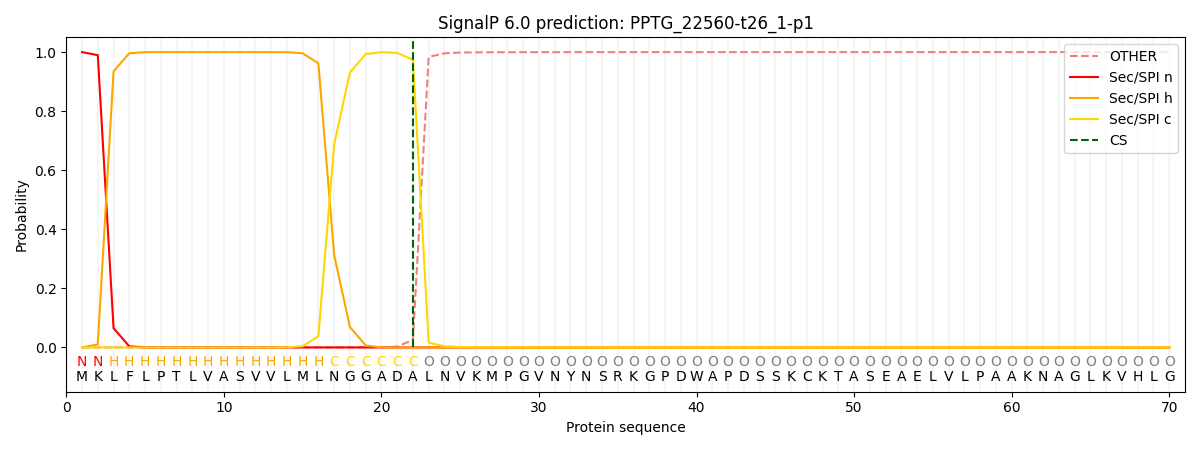
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000291 | 0.999664 | CS pos: 22-23. Pr: 0.9743 |
