You are browsing environment: FUNGIDB
CAZyme Information: PPTG_10389-t26_1-p1
You are here: Home > Sequence: PPTG_10389-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phytophthora parasitica | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora parasitica | |||||||||||
| CAZyme ID | PPTG_10389-t26_1-p1 | |||||||||||
| CAZy Family | GH30 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT41 | 60 | 746 | 4.4e-112 | 0.6595744680851063 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 226428 | Spy | 4.43e-92 | 166 | 745 | 63 | 614 | Predicted O-linked N-acetylglucosamine transferase, SPINDLY family [Posttranslational modification, protein turnover, chaperones]. |
| 404688 | Glyco_transf_41 | 2.11e-63 | 290 | 735 | 2 | 540 | Glycosyl transferase family 41. This family of glycosyltransferases includes O-linked beta-N-acetylglucosamine (O-GlcNAc) transferase, an enzyme which catalyzes the addition of O-GlcNAc to serine and threonine residues. In addition to its function as an O-GlcNAc transferase, human OGT also appears to proteolytically cleave the epigenetic cell-cycle regulator HCF-1. |
| 340831 | GT4_PimA-like | 3.14e-19 | 1023 | 1385 | 2 | 365 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| 276809 | TPR | 2.74e-16 | 791 | 882 | 5 | 96 | Tetratricopeptide repeat. The Tetratricopeptide repeat (TPR) typically contains 34 amino acids and is found in a variety of organisms including bacteria, cyanobacteria, yeast, fungi, plants, and humans. It is present in a variety of proteins including those involved in chaperone, cell-cycle, transcription, and protein transport complexes. The number of TPR motifs varies among proteins. Those containing 5-6 tandem repeats generate a right-handed helical structure with an amphipathic channel that is thought to accommodate an alpha-helix of a target protein. It has been proposed that TPR proteins preferentially interact with WD-40 repeat proteins, but in many instances several TPR-proteins seem to aggregate to multi-protein complexes. |
| 223515 | RfaB | 2.28e-15 | 1104 | 1387 | 109 | 376 | Glycosyltransferase involved in cell wall bisynthesis [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 4.31e-91 | 42 | 584 | 21 | 559 | |
| 2.84e-84 | 375 | 748 | 23 | 380 | |
| 3.78e-84 | 282 | 748 | 176 | 617 | |
| 8.73e-83 | 375 | 745 | 16 | 370 | |
| 1.81e-81 | 282 | 748 | 583 | 1024 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.91e-53 | 290 | 751 | 159 | 714 | Crystal structure of the O-GlcNAc transferase Asn648Tyr mutation [Homo sapiens] |
|
| 6.48e-53 | 290 | 751 | 153 | 708 | The human O-GlcNAc transferase in complex with a thiol-linked bisubstrate inhibitor [Homo sapiens] |
|
| 6.57e-53 | 290 | 751 | 154 | 709 | The human O-GlcNAc transferase in complex with a bisubstrate inhibitor [Homo sapiens] |
|
| 7.03e-53 | 290 | 751 | 159 | 714 | Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_B Chain B, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_D Chain D, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE4_A Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3PE4_C Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3TAX_A A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],3TAX_C A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],4AY5_A Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_B Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_C Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_D Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY6_A Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_B Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_C Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_D Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4CDR_A Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_B Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_C Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_D Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4GYW_A Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYW_C Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYY_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GYY_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GZ3_A Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ3_C Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ5_A Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_B Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_C Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_D Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ6_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_B Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_D Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4N39_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) [Homo sapiens],4N3A_A Crystal Structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (1-26)E10A [Homo sapiens],4N3B_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26)E10Q and UDP-5SGlcNAc [Homo sapiens],4N3C_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26) and UDP-GlcNAc [Homo sapiens],4XI9_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XIF_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],5BNW_A The active site of O-GlcNAc transferase imposes constraints on substrate sequence [Homo sapiens],5C1D_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RB2L) [Homo sapiens],5VIE_A Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIE_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIF_A Electrophilic probes for deciphering substrate recognition by O-GlcNAc transferase [Homo sapiens],6E37_A O-GlcNAc Transferase in complex with covalent inhibitor [Homo sapiens],6MA1_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 4a [Homo sapiens],6MA2_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor ent-1a [Homo sapiens],6MA3_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 2a [Homo sapiens],6MA4_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 3a [Homo sapiens],6MA5_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 1a [Homo sapiens] |
|
| 7.42e-53 | 290 | 751 | 158 | 713 | Chain AAA, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.83e-59 | 282 | 742 | 501 | 948 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SEC OS=Arabidopsis thaliana OX=3702 GN=SEC PE=1 SV=1 |
|
| 1.04e-51 | 290 | 751 | 477 | 1032 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Mus musculus OX=10090 GN=Ogt PE=1 SV=2 |
|
| 4.21e-51 | 290 | 751 | 477 | 1032 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Oryctolagus cuniculus OX=9986 GN=OGT PE=1 SV=2 |
|
| 5.57e-51 | 290 | 751 | 477 | 1032 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Homo sapiens OX=9606 GN=OGT PE=1 SV=3 |
|
| 1.29e-50 | 290 | 751 | 477 | 1032 | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit OS=Sus scrofa OX=9823 GN=OGT PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
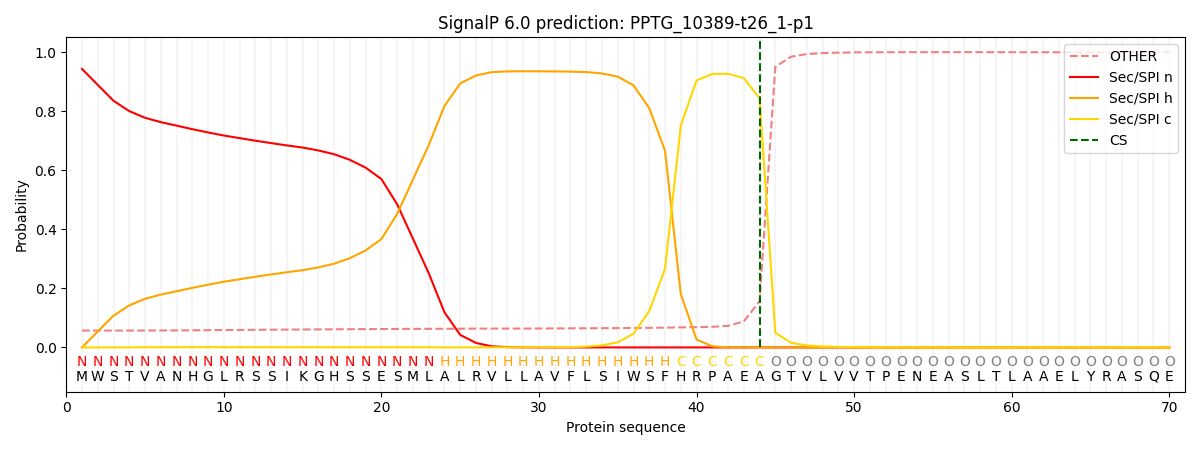
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.063933 | 0.936066 | CS pos: 44-45. Pr: 0.8436 |
