You are browsing environment: FUNGIDB
CAZyme Information: PPTG_04233-t26_1-p1
You are here: Home > Sequence: PPTG_04233-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phytophthora parasitica | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora parasitica | |||||||||||
| CAZyme ID | PPTG_04233-t26_1-p1 | |||||||||||
| CAZy Family | CBM48 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.-:29 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT24 | 1377 | 1622 | 5.3e-122 | 0.9959677419354839 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 408203 | Glyco_transf_24 | 0.0 | 1377 | 1647 | 1 | 267 | Glucosyltransferase 24. This is the catalytic domain found in UDP-glucose:glycoprotein glucosyltransferase (UGGT). This domain belongs to glucosyltransferase 24 family (GT24) A-type domain. The GT domain displays the expected glycosyltransferase type A (GT-A) fold. |
| 133054 | GT8_HUGT1_C_like | 8.06e-161 | 1377 | 1622 | 1 | 248 | The C-terminal domain of HUGT1-like is highly homologous to the GT 8 family. C-terminal domain of glycoprotein glucosyltransferase (UGT). UGT is a large glycoprotein whose C-terminus contains the catalytic activity. This catalytic C-terminal domain is highly homologous to Glycosyltransferase Family 8 (GT 8) and contains the DXD motif that coordinates donor sugar binding, characteristic for Family 8 glycosyltransferases. GT 8 proteins are retaining enzymes based on the relative anomeric stereochemistry of the substrate and product in the reaction catalyzed. The non-catalytic N-terminal portion of the human UTG1 (HUGT1) has been shown to monitor the protein folding status and activate its glucosyltransferase activity. |
| 132996 | Glyco_transf_8 | 1.43e-57 | 1377 | 1617 | 1 | 243 | Members of glycosyltransferase family 8 (GT-8) are involved in lipopolysaccharide biosynthesis and glycogen synthesis. Members of this family are involved in lipopolysaccharide biosynthesis and glycogen synthesis. GT-8 comprises enzymes with a number of known activities: lipopolysaccharide galactosyltransferase, lipopolysaccharide glucosyltransferase 1, glycogenin glucosyltransferase, and N-acetylglucosaminyltransferase. GT-8 enzymes contains a conserved DXD motif which is essential in the coordination of a catalytic divalent cation, most commonly Mn2+. |
| 408199 | Thioredoxin_12 | 1.40e-35 | 38 | 243 | 1 | 182 | Thioredoxin-like domain. This is one of four TRXL(thioredoxin-like) domains found in UDP-glucose:glycoprotein glucosyltransferase (UGGT). |
| 399437 | UDP-g_GGTase | 5.64e-28 | 1201 | 1308 | 2 | 109 | UDP-glucose:Glycoprotein Glucosyltransferase. UDP-g_GGTase is an important, central component of the QC system in the ER for checking that glycoproteins are folded correctly. This QC prevents incorrectly folded glycoproteins from leaving the ER. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 1657 | 1 | 1665 | |
| 0.0 | 26 | 1654 | 36 | 1667 | |
| 0.0 | 11 | 1654 | 11 | 1507 | |
| 0.0 | 21 | 1653 | 19 | 1516 | |
| 5.62e-204 | 26 | 1656 | 34 | 1563 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.78e-147 | 205 | 1647 | 164 | 1446 | Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],5MZO_A Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],5N2J_A Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],5N2J_B Chain B, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],6TRF_A Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495] |
|
| 3.82e-147 | 205 | 1647 | 164 | 1446 | Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495] |
|
| 5.25e-147 | 205 | 1647 | 164 | 1446 | Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495] |
|
| 2.73e-141 | 812 | 1647 | 397 | 1212 | Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein,UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],6TS2_B Chain B, UDP-glucose-glycoprotein glucosyltransferase-like protein,UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],6TS2_C Chain C, UDP-glucose-glycoprotein glucosyltransferase-like protein,UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],6TS2_D Chain D, UDP-glucose-glycoprotein glucosyltransferase-like protein,UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495] |
|
| 2.90e-121 | 205 | 1584 | 157 | 1382 | Chain A, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495],6TS8_B Chain B, UDP-glucose-glycoprotein glucosyltransferase-like protein [Thermochaetoides thermophila DSM 1495] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.25e-190 | 26 | 1651 | 31 | 1571 | UDP-glucose:glycoprotein glucosyltransferase OS=Arabidopsis thaliana OX=3702 GN=UGGT PE=1 SV=1 |
|
| 1.98e-185 | 21 | 1646 | 28 | 1496 | UDP-glucose:glycoprotein glucosyltransferase 2 OS=Homo sapiens OX=9606 GN=UGGT2 PE=1 SV=4 |
|
| 5.72e-184 | 2 | 1652 | 16 | 1527 | UDP-glucose:glycoprotein glucosyltransferase 1 OS=Mus musculus OX=10090 GN=Uggt1 PE=1 SV=4 |
|
| 2.24e-181 | 26 | 1652 | 45 | 1527 | UDP-glucose:glycoprotein glucosyltransferase 1 OS=Homo sapiens OX=9606 GN=UGGT1 PE=1 SV=3 |
|
| 2.00e-179 | 24 | 1652 | 43 | 1527 | UDP-glucose:glycoprotein glucosyltransferase 1 OS=Rattus norvegicus OX=10116 GN=Uggt1 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
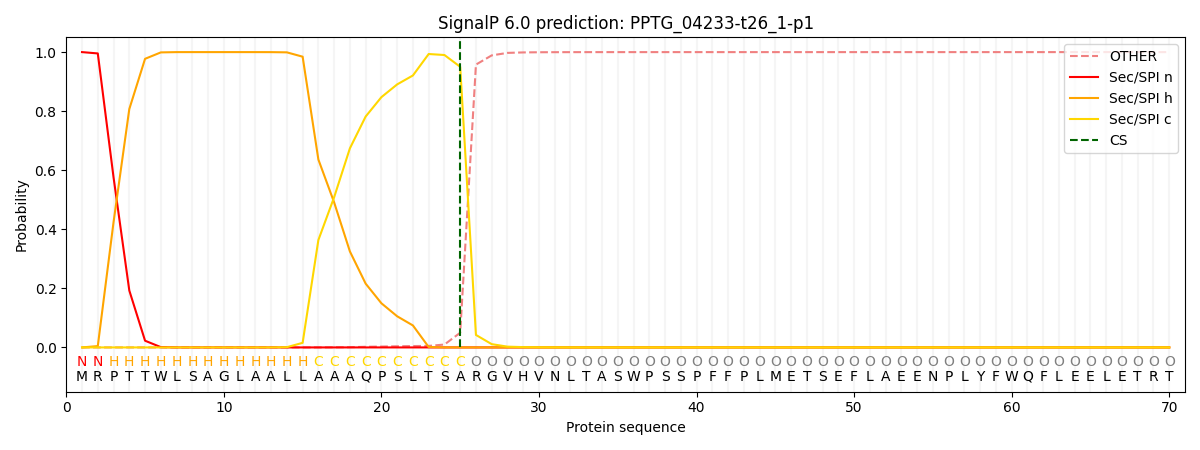
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000250 | 0.999718 | CS pos: 25-26. Pr: 0.9503 |
