You are browsing environment: FUNGIDB
CAZyme Information: PNEJI1_000567-t26_1-p1
You are here: Home > Sequence: PNEJI1_000567-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
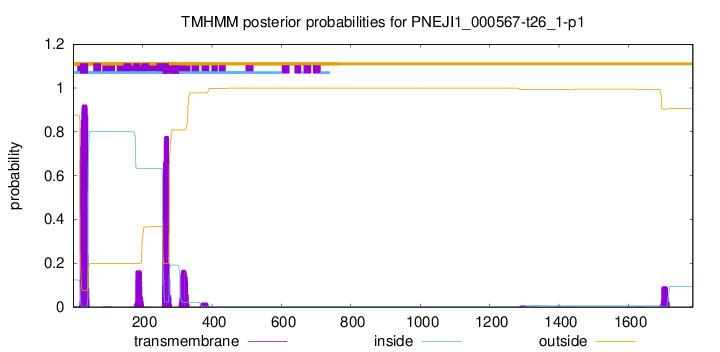
TMHMM annotations
Basic Information help
| Species | Pneumocystis jirovecii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Pneumocystidomycetes; ; Pneumocystidaceae; Pneumocystis; Pneumocystis jirovecii | |||||||||||
| CAZyme ID | PNEJI1_000567-t26_1-p1 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.25:2 | 3.2.1.33:2 | 2.4.1.25:22 | 3.2.1.33:21 | 2.4.1.25:22 | 3.2.1.33:21 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 1350 | 1773 | 9.8e-177 | 0.9930875576036866 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 200466 | AmyAc_Glg_debranch_2 | 0.0 | 419 | 902 | 1 | 478 | Alpha amylase catalytic domain found in glycogen debranching enzymes. Debranching enzymes facilitate the breakdown of glycogen through glucosyltransferase and glucosidase activity. These activities are performed by a single enzyme in mammals, yeast, and some bacteria, but by two distinct enzymes in Escherichia coli and other bacteria. Debranching enzymes perform two activities, 4-alpha-D-glucanotransferase (EC 2.4.1.25) and amylo-1,6-glucosidase (EC 3.2.1.33). 4-alpha-D-glucanotransferase catalyzes the endohydrolysis of 1,6-alpha-D-glucoside linkages at points of branching in chains of 1,4-linked alpha-D-glucose residues. Amylo-alpha-1,6-glucosidase catalyzes the endohydrolysis of 1,6-alpha-D-glucoside linkages at points of branching in chains of 1,4-linked alpha-D-glucose residues. The catalytic triad (DED), which is highly conserved in other debranching enzymes, is not present in this group. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| 273673 | glyc_debranch | 0.0 | 349 | 1779 | 42 | 1464 | glycogen debranching enzymye. glycogen debranching enzyme possesses two different catalytic activities; oligo-1,4-->1,4-glucantransferase (EC 2.4.1.25) and amylo-1,6-glucosidase (EC 3.2.1.33). Site directed mutagenesis studies in S. cerevisiae indicate that the transferase and glucosidase activities are independent and located in different regions of the polypeptide chain. Proteins in this model belong to the larger alpha-amylase family. The model covers eukaryotic proteins with a seed composed of human, nematode and yeast sequences. Yeast seed sequence is well characterized. The model is quite rigorous; either query sequence yields large bit score or it fails to hit the model altogether. There doesn't appear to be any middle ground. [Energy metabolism, Biosynthesis and degradation of polysaccharides] |
| 405401 | hDGE_amylase | 0.0 | 435 | 870 | 3 | 439 | Glycogen debranching enzyme, glucanotransferase domain. This is a glucanotransferase catalytic domain of the eukaryotic variant of the glycogen debranching enzyme (GDE). The eukaryotic GDEs performs two functions: 4-alpha-D-glucanotransferase, EC:2.4.1.25, and Amylo-alpha-1,6-glucosidase, EC:3.2.1.33, performed by the, respectively N- and C- terminal halves of eukaryotic GDE enzymes. The domain is a catalytic domain responsible for the glucanotransferase function. It belongs to the alpha-amylase clan and is predicted to have a structure of a 8-stranded alpha/beta barrel (TIM barrel) where strands are interrupted by long loops and additional mini-domains. In most other amylases, the catalytic domain is followed by a beta- barrel substrate binding domain, but presence of such a domain cannot be verified in the human (and other eukaryotic) GDE enzymes. |
| 396646 | Cytochrom_C1 | 4.59e-128 | 66 | 284 | 1 | 219 | Cytochrome C1 family. |
| 405402 | hGDE_central | 1.52e-114 | 1022 | 1258 | 1 | 242 | Central domain of human glycogen debranching enzyme. This is a central domain of the eukaryotic variant of the glycogen debranching enzyme (GDE). The eukaryotic GDE performs two functions: 4-alpha-D-glucanotransferase, EC:2.4.1.25, and Amylo-alpha-1,6-glucosidase, EC:3.2.1.33, performed by the, respectively N- and C- terminal halves of eukaryotic GDE enzyme This central domain follows the glucanotransferase domain and precedes the glucosidase (GDE_N) domain. It is very likely that the current definition contains two or more domains, by analogy with baterial GDEs, this domain should be involved in substrate- binding either for the N-terminal glucanotransferase and/or the the C-terminal glucosidase (or both). |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 300 | 1782 | 1 | 1437 | |
| 0.0 | 300 | 1780 | 1 | 1513 | |
| 0.0 | 300 | 1786 | 1 | 1548 | |
| 0.0 | 300 | 1786 | 1 | 1548 | |
| 0.0 | 300 | 1786 | 1 | 1548 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 310 | 1779 | 28 | 1522 | Crystal Structure of the Candida Glabrata Glycogen Debranching Enzyme (E564Q) in complex with maltopentaose [[Candida] glabrata CBS 138],5D0F_B Crystal Structure of the Candida Glabrata Glycogen Debranching Enzyme (E564Q) in complex with maltopentaose [[Candida] glabrata CBS 138] |
|
| 0.0 | 310 | 1779 | 28 | 1522 | Chain A, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138],7EKX_B Chain B, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138] |
|
| 0.0 | 310 | 1779 | 28 | 1522 | Chain A, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138],7EKW_B Chain B, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138] |
|
| 0.0 | 310 | 1779 | 28 | 1522 | Crystal Structure of the Candida Glabrata Glycogen Debranching Enzyme [[Candida] glabrata CBS 138],5D06_B Crystal Structure of the Candida Glabrata Glycogen Debranching Enzyme [[Candida] glabrata CBS 138] |
|
| 0.0 | 310 | 1779 | 28 | 1522 | Chain A, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138],7EKU_B Chain B, 4-alpha-glucanotransferase [[Candida] glabrata CBS 138] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 308 | 1779 | 7 | 1528 | Glycogen debranching enzyme OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=GDB1 PE=1 SV=1 |
|
| 8.75e-302 | 353 | 1777 | 49 | 1532 | Glycogen debranching enzyme OS=Equus caballus OX=9796 GN=AGL PE=2 SV=1 |
|
| 5.93e-298 | 353 | 1777 | 49 | 1531 | Glycogen debranching enzyme OS=Homo sapiens OX=9606 GN=AGL PE=1 SV=3 |
|
| 9.28e-297 | 353 | 1777 | 49 | 1532 | Glycogen debranching enzyme OS=Canis lupus familiaris OX=9615 GN=AGL PE=2 SV=1 |
|
| 1.45e-294 | 353 | 1777 | 72 | 1554 | Glycogen debranching enzyme OS=Oryctolagus cuniculus OX=9986 GN=AGL PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
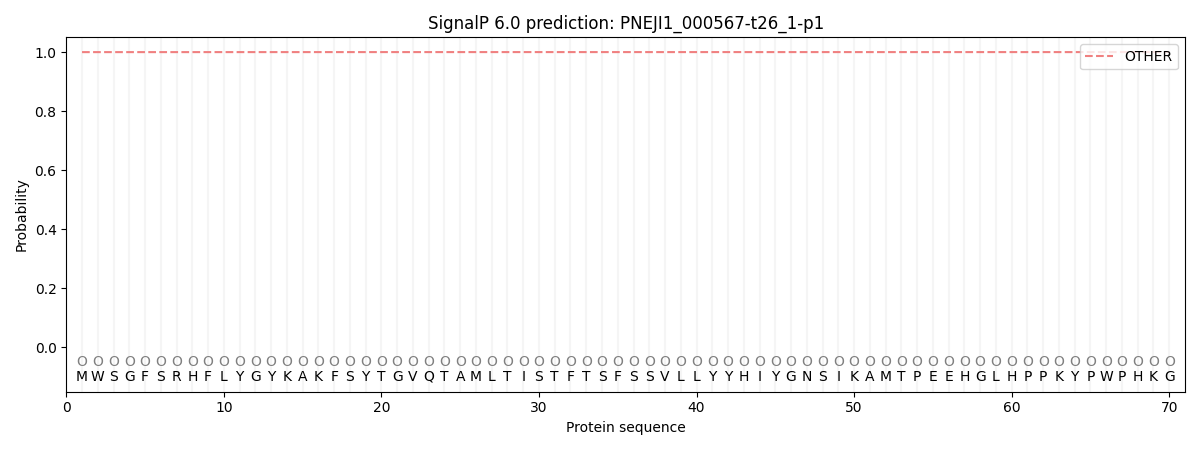
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000018 | 0.000011 |