You are browsing environment: FUNGIDB
CAZyme Information: PMAA_100200-t26_1-p1
You are here: Home > Sequence: PMAA_100200-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Talaromyces marneffei | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Trichocomaceae; Talaromyces; Talaromyces marneffei | |||||||||||
| CAZyme ID | PMAA_100200-t26_1-p1 | |||||||||||
| CAZy Family | GT32 | |||||||||||
| CAZyme Description | integral membrane protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA8 | 21 | 186 | 3.9e-19 | 0.9120879120879121 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 187688 | CDH_like_cytochrome | 3.47e-50 | 13 | 182 | 2 | 168 | Heme-binding cytochrome domain of fungal cellobiose dehydrogenases. Cellobiose dehydrogenase (CellobioseDH or CDH) is an extracellular fungal oxidoreductase that degrades both lignin and cellulose. Specifically, CDHs oxidize cellobiose, cellodextrins, and lactose to corresponding lactones, utilizing a variety of electron acceptors. Class-II CDHs are monomeric hemoflavoenzymes that are comprised of a b-type cytochrome domain linked to a large flavodehydrogenase domain. The cytochrome domain of CDH and related enzymes, which this model describes, folds as a beta sandwich and complexes a heme molecule. It is found at the N-terminus of this family of enzymes, and belongs to the DOMON domain superfamily, a ligand-interacting motif found in all three kingdoms of life. |
| 406418 | CDH-cyt | 1.40e-30 | 22 | 186 | 10 | 174 | Cytochrome domain of cellobiose dehydrogenase. CDH-cyt is the cytochrome domain, at the N-terminus, of cellobiose dehydrogenase. CDH-cyt folds as a beta sandwich with the topology of the antibody Fab V(H) domain and binds iron. The haem iron is ligated by Met83 and His181 in UniProtKB:Q01738. |
| 176490 | Cyt_b561_FRRS1_like | 1.35e-26 | 228 | 372 | 26 | 168 | Eukaryotic cytochrome b(561), including the FRRS1 gene product. Cytochrome b(561), as found in eukaryotes, similar to and including the human FRRS1 gene product (ferric-chelate reductase 1), also called SDR-2 (stromal cell-derived receptor 2). This family comprises a variety of domain architectures, many of which contain dopamine beta-monooxygenase (DOMON) domains. The protein might act as a ferric-chelate reductase, catalyzing the reduction of Fe(3+) to Fe(2+), such as associated with the transport of iron from the endosome to the cytoplasm. It is assumed that this protein uses ascorbate as the electron donor. Belongs to the cytochrome b(561) family, which are secretory vesicle-specific electron transport proteins. Cytochromes b(561) are integral membrane proteins that bind two heme groups non-covalently, and may have six alpha-helical trans-membrane segments. |
| 214769 | B561 | 1.96e-10 | 241 | 364 | 2 | 129 | Cytochrome b-561 / ferric reductase transmembrane domain. Cytochrome b-561 recycles ascorbate for the generation of norepinephrine by dopamine-beta-hydroxylase in the chromaffin vesicles of the adrenal gland. It is a transmembrane heme protein with the two heme groups being bound to conserved histidine residues. A cytochrome b-561 homologue, termed Dcytb, is an iron-regulated ferric reductase in the duodenal mucosa. Other homologues of these are also likely to be ferric reductases. SDR2 is proposed to be important in regulating the metabolism of iron in the onset of neurodegenerative disorders. |
| 176496 | Cyt_b561_ACYB-1_like | 1.77e-05 | 233 | 364 | 1 | 136 | Plant cytochrome b(561), including the carbon monoxide oxygenase ACYB-1. Cytochrome b(561), as found in plants, similar to the Arabidopsis thaliana ACYB-1 gene product, a cytochrome b561 isoform localized to the tonoplast. This protein might act as a ferric-chelate reductase, catalyzing the reduction of Fe(3+) to Fe(2+), and might be capable of trans-membrane electron transport from intracellular ascorbate to extracellular ferric chelates. It is assumed that this protein uses ascorbate as the electron donor. Belongs to the cytochrome b(561) family, which are secretory vesicle-specific electron transport proteins. Cytochromes b(561) are integral membrane proteins that bind two heme groups non-covalently, and may have six alpha-helical trans-membrane segments. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.53e-62 | 12 | 393 | 11 | 374 | |
| 1.53e-62 | 12 | 393 | 11 | 374 | |
| 3.51e-60 | 12 | 394 | 12 | 374 | |
| 4.80e-57 | 33 | 414 | 25 | 375 | |
| 4.80e-57 | 33 | 414 | 25 | 375 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
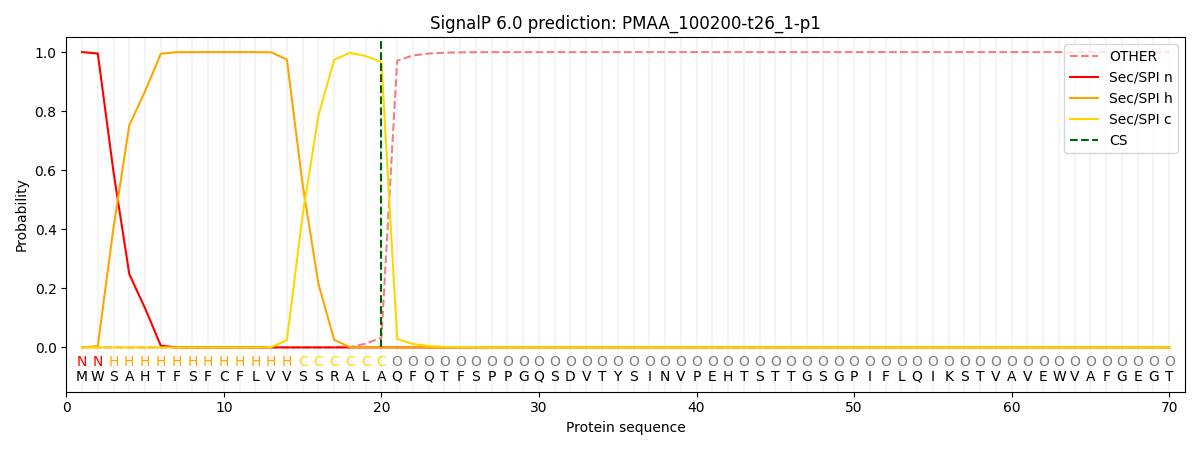
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000234 | 0.999727 | CS pos: 20-21. Pr: 0.9668 |
TMHMM Annotations download full data without filtering help

| Start | End |
|---|---|
| 242 | 264 |
| 271 | 293 |
| 308 | 327 |
| 347 | 369 |
| 379 | 401 |
