You are browsing environment: FUNGIDB
CAZyme Information: PIW_T001662-RA-p1
You are here: Home > Sequence: PIW_T001662-RA-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Globisporangium iwayamae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Pythiaceae; Globisporangium; Globisporangium iwayamae | |||||||||||
| CAZyme ID | PIW_T001662-RA-p1 | |||||||||||
| CAZy Family | AA17 | |||||||||||
| CAZyme Description | Multicopper oxidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 39 | 536 | 1.9e-92 | 0.9804469273743017 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 274555 | ascorbase | 1.06e-68 | 25 | 542 | 4 | 524 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
| 177843 | PLN02191 | 2.02e-62 | 25 | 548 | 26 | 553 | L-ascorbate oxidase |
| 215324 | PLN02604 | 3.09e-60 | 25 | 542 | 27 | 547 | oxidoreductase |
| 274556 | laccase | 2.40e-53 | 31 | 540 | 7 | 518 | laccase, plant. Members of this protein family include the copper-containing enzyme laccase (EC 1.10.3.2), often several from a single plant species, and additional, uncharacterized, closely related plant proteins termed laccase-like multicopper oxidases. This protein family shows considerable sequence similarity to the L-ascorbate oxidase (EC 1.10.3.3) family. Laccases are enzymes of rather broad specificity, and classification of all proteins scoring about the trusted cutoff of this model as laccases may be appropriate. |
| 225043 | SufI | 1.89e-52 | 37 | 536 | 48 | 443 | Multicopper oxidase with three cupredoxin domains (includes cell division protein FtsP and spore coat protein CotA) [Cell cycle control, cell division, chromosome partitioning, Inorganic ion transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 9.12e-166 | 25 | 562 | 26 | 561 | |
| 3.10e-164 | 2 | 563 | 5 | 545 | |
| 6.29e-164 | 1 | 559 | 1 | 561 | |
| 2.51e-153 | 16 | 563 | 10 | 537 | |
| 3.47e-103 | 24 | 562 | 43 | 554 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.02e-62 | 37 | 549 | 82 | 550 | Crystal structure of laccase from Botrytis aclada at 1.67 A resolution [Botrytis aclada],4X4K_A Structure of laccase from Botrytis aclada with full copper content [Botrytis aclada] |
|
| 7.77e-62 | 37 | 549 | 82 | 550 | Structure of the L499M mutant of the laccase from B.aclada [Botrytis aclada] |
|
| 4.36e-58 | 29 | 541 | 11 | 468 | Chain A, LACCASE 1 [Coprinopsis cinerea] |
|
| 4.46e-58 | 29 | 541 | 11 | 468 | Chain A, Laccase [Coprinopsis cinerea] |
|
| 2.66e-57 | 33 | 542 | 14 | 466 | Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena],5Z1X_B Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena],5Z22_A Crystal Structure of Laccase from Cerrena sp. RSD1 [Cerrena] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.29e-63 | 25 | 561 | 64 | 581 | Laccase-1 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC1 PE=1 SV=1 |
|
| 1.50e-63 | 37 | 546 | 83 | 548 | Laccase-2 OS=Botryotinia fuckeliana OX=40559 GN=lcc2 PE=2 SV=1 |
|
| 1.79e-63 | 25 | 541 | 64 | 561 | Laccase-1 OS=Cryptococcus neoformans var. neoformans serotype D (strain B-3501A) OX=283643 GN=LAC1 PE=1 SV=1 |
|
| 3.76e-63 | 25 | 549 | 64 | 567 | Laccase-2 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC2 PE=3 SV=2 |
|
| 2.23e-61 | 20 | 545 | 19 | 491 | Laccase-1 OS=Agaricus bisporus OX=5341 GN=lcc1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
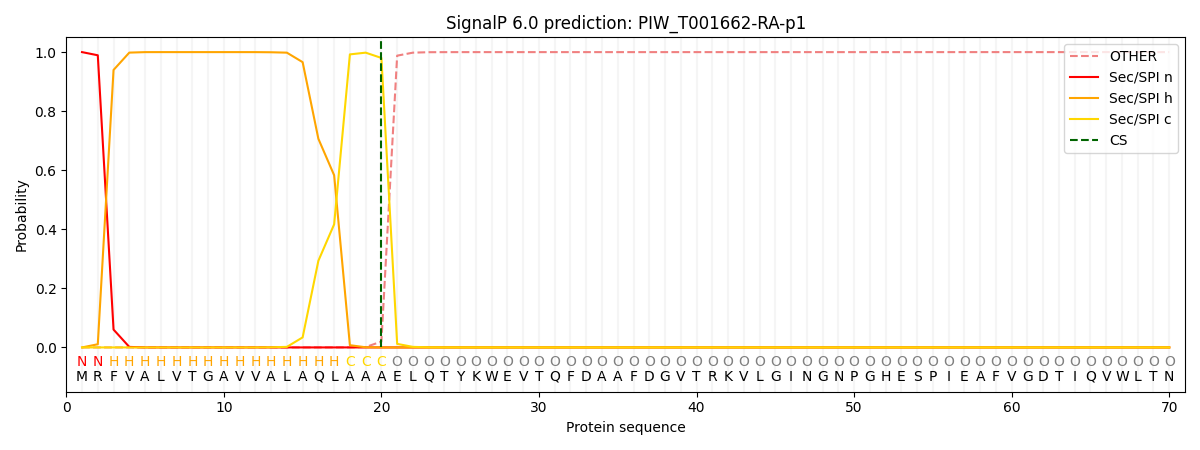
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000208 | 0.999751 | CS pos: 20-21. Pr: 0.9802 |
