You are browsing environment: FUNGIDB
CAZyme Information: PHYSODRAFT_337880-t26_1-p1
You are here: Home > Sequence: PHYSODRAFT_337880-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phytophthora sojae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Peronosporaceae; Phytophthora; Phytophthora sojae | |||||||||||
| CAZyme ID | PHYSODRAFT_337880-t26_1-p1 | |||||||||||
| CAZy Family | GH17 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH141 | 742 | 989 | 1.6e-20 | 0.45540796963946867 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 187737 | Peptidase_C12_UCH_L1_L3 | 2.01e-88 | 106 | 329 | 1 | 222 | Cysteine peptidase C12 containing ubiquitin carboxyl-terminal hydrolase (UCH) families L1 and L3. This ubiquitin C-terminal hydrolase (UCH) family includes UCH-L1 and UCH-L3, the two members sharing around 53% sequence identity as well as conserved catalytic residues. Both enzymes hydrolyze carboxyl terminal esters and amides of ubiquitin (Ub). UCH-L1, in dimeric form, has additional enzymatic activity as a ubiquitin ligase. It is highly abundant in the brain, constituting up to 2% of total protein, and is expressed exclusively in neurons and testes. Abnormal expression of UCH-L1 has been shown to correlate with several forms of cancer, including several primary lung tumors, lung tumor cell lines, and colorectal cancers. Mutations in the UCH-L1 gene have been linked to susceptibility to and protection from Parkinson's disease (PD); dysfunction of the hydrolase activity can lead to an accumulation of alpha-synuclein, which is linked to Parkinson's disease (PD), while accumulation of neurofibrillary tangles is linked to Alzheimer's disease (AD). UCH-L3 hydrolyzes isopeptide bonds at the C-terminal glycine of either Ub or Nedd8, a ubiquitin-like protein. It can also interact with Lys48-linked Ub dimers to protect them from degradation while inhibiting its hydrolase activity at the same time. Unlike UCH-L1, neither dimerization nor ligase activity have been observed for UCH-L3. It has been shown that levels of Nedd8 and the apoptotic protein p53 and Bax are elevated in UCH-L3 knockout mice upon cryptorchid injury, possibly contributing to profound germ cell loss via apoptosis. |
| 395865 | Peptidase_C12 | 2.59e-76 | 108 | 316 | 2 | 205 | Ubiquitin carboxyl-terminal hydrolase, family 1. |
| 187738 | Peptidase_C12_UCH37_BAP1 | 2.30e-23 | 106 | 329 | 1 | 219 | Cysteine peptidase C12 containing ubiquitin carboxyl-terminal hydrolase (UCH) families UCH37 (UCH-L5) and BAP1. This ubiquitin C-terminal hydrolase (UCH) family includes UCH37 (also known as UCH-L5) and BRCA1-associated protein-1 (BAP1). They contain a UCH catalytic domain as well as an additional C-terminal extension which plays a role in protein-protein interactions. UCH37 is responsible for ubiquitin (Ub) isopeptidase activity in the 19S proteasome regulatory complex; it disassembles Lys48-linked poly-ubiquitin from the distal end of the chain. It is also associated with the human Ino80 chromatin-remodeling complex (hINO80) in the nucleus and can be activated through transient association of hINO80 with hRpn13 that is bound to the 19S regulatory particle or the proteasome. UCH37 possibly plays a role in oncogenesis; it competes with Smad ubiquitination regulatory factor 2 (Smurf2, ubiquitin ligase) in binding concurrently to Smad7 in order to deubiquitinate the activated type I transforming growth factor beta (TGF-beta) receptor, thus rescuing it from proteasomal degradation. BAP1 binds to the wild-type BRCA1 RING finger domain, localized in the nucleus. In addition to the UCH catalytic domain, BAP1 contains a UCH37-like domain (ULD), binding domains for BRCA1 and BARD1, which form a tumor suppressor heterodimeric complex, and a binding domain for HCFC1, which interacts with histone-modifying complexes during cell division. The full-length human BRCA1 is a ubiquitin ligase. However, BAP1 does not appear to function in the deubiquitination of autoubiquitinated BRCA1. BAP1 exhibits tumor suppressor activity in cancer cells, and gene mutations have been reported in a small number of breast and lung cancer samples. In metastasis of uveal melanoma, the most common primary cancer of the eye, inactivating somatic mutations have been identified in the gene encoding BAP1 on chromosome 3p21.1. These mutations include several that cause premature protein termination as well as affect its UCH domain, thus implicating loss of BAP1 and suggesting that the BAP1 pathway may be a valuable therapeutic target. |
| 187736 | Peptidase_C12 | 2.87e-13 | 106 | 329 | 1 | 222 | Cysteine peptidase C12 contains ubiquitin carboxyl-terminal hydrolase (UCH) families L1, L3, L5 and BAP1. The ubiquitin C-terminal hydrolase (UCH; ubiquitinyl hydrolase; ubiquitin thiolesterase) family of deubiquitinating enzymes (DUBs) consists of four members to date: UCH-L1, UCH-L3, UCH-L5 (UCH37) and BRCA1-associated protein-1 (BAP1), all containing a conserved catalytic domain with cysteine peptidase activity. UCH-L1 hydrolyzes carboxyl terminal esters and amides of ubiquitin (Ub). Dysfunction of this hydrolase activity can lead to an accumulation of alpha-synuclein, which is linked to Parkinson's disease (PD) and neurofibrillary tangles, linked to Alzheimer's disease (AD). UCH-L1, in its dimeric form, has additional enzymatic activity as a ubiquitin ligase. UCH-L3 hydrolyzes isopeptide bonds at the C-terminal glycine of either Ub or Nedd8, a ubiquitin-like protein. UCH-L3 can also interact with Lys48-linked Ub dimers to protect it from degradation while inhibiting its hydrolase activity at the same time. UCH-L1 and UCH-L3 are the most closely related of the UCH members. UCH-L5 (UCH37) is involved in the deubiquitinating activity in the 19S proteasome regulatory complex. It is also associated with the human Ino80 chromatin-remodeling complex (hINO80) in the nucleus. BAP1 binds to the wild-type BRCA1 RING finger domain, localized in the nucleus. It consists of the N-terminal UCH domain and two predicted nuclear localization signals (NLSs), only one of which is functional. The full-length human BRCA1 is a ubiquitin ligase. However, BAP1 does not appear to function in the deubiquitination of autoubiquitinated BRCA1. There is growing evidence that UCH enzymes and human malignancies are closely correlated. Studies show that UCH enzymes play a crucial role in some signaling pathways and in cell-cycle regulation. |
| 404168 | Beta_helix | 8.36e-05 | 874 | 985 | 1 | 110 | Right handed beta helix region. This region contains a parallel beta helix region that shares some similarity with Pectate lyases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.20e-15 | 716 | 991 | 148 | 381 | |
| 8.10e-15 | 690 | 991 | 343 | 601 | |
| 3.14e-12 | 716 | 991 | 319 | 552 | |
| 3.28e-12 | 716 | 991 | 375 | 608 | |
| 1.32e-11 | 716 | 991 | 410 | 643 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.14e-60 | 104 | 330 | 1 | 225 | UCHL3 in complex with synthetic, K27-linked diubiquitin [Homo sapiens],6QML_D UCHL3 in complex with synthetic, K27-linked diubiquitin [Homo sapiens] |
|
| 1.25e-60 | 104 | 330 | 4 | 228 | Deubiquitinating Enzyme Uch-L3 (Human) At 1.8 Angstrom Resolution [Homo sapiens],1XD3_A Crystal structure of UCHL3-UbVME complex [Homo sapiens],1XD3_C Crystal structure of UCHL3-UbVME complex [Homo sapiens],6ISU_A Crystal structure of Lys27-linked di-ubiquitin in complex with its selective interacting protein UCHL3 [Homo sapiens] |
|
| 1.98e-57 | 108 | 333 | 10 | 228 | Crystal Structure of Ubiquitin Carboxy-terminal Hydrolase L1 (UCH-L1) [Homo sapiens],2ETL_B Crystal Structure of Ubiquitin Carboxy-terminal Hydrolase L1 (UCH-L1) [Homo sapiens],3KW5_A Crystal structure of ubiquitin carboxy terminal hydrolase L1 bound to ubiquitin vinylmethylester [Homo sapiens],4DM9_A The Crystal Structure of Ubiquitin Carboxy-terminal hydrolase L1 (UCHL1) bound to a tripeptide fluoromethyl ketone Z-VAE(OMe)-FMK [Homo sapiens],4DM9_B The Crystal Structure of Ubiquitin Carboxy-terminal hydrolase L1 (UCHL1) bound to a tripeptide fluoromethyl ketone Z-VAE(OMe)-FMK [Homo sapiens] |
|
| 3.68e-57 | 108 | 333 | 10 | 228 | Chain A, Ubiquitin carboxyl-terminal hydrolase isozyme L1 [Homo sapiens],3IRT_B Chain B, Ubiquitin carboxyl-terminal hydrolase isozyme L1 [Homo sapiens],3KVF_A Chain A, Ubiquitin carboxyl-terminal hydrolase isozyme L1 [Homo sapiens] |
|
| 3.68e-57 | 108 | 333 | 10 | 228 | Chain A, Ubiquitin carboxyl-terminal hydrolase isozyme L1 [Homo sapiens],4JKJ_A Crystal Structure of the S18Y Variant of Ubiquitin Carboxy-terminal Hydrolase L1 [Homo sapiens],4JKJ_B Crystal Structure of the S18Y Variant of Ubiquitin Carboxy-terminal Hydrolase L1 [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.57e-65 | 97 | 331 | 4 | 232 | Ubiquitin carboxyl-terminal hydrolase 3 OS=Arabidopsis thaliana OX=3702 GN=UCH3 PE=2 SV=1 |
|
| 1.87e-60 | 104 | 330 | 4 | 228 | Ubiquitin carboxyl-terminal hydrolase isozyme L3 OS=Sus scrofa OX=9823 GN=UCHL3 PE=2 SV=1 |
|
| 6.45e-60 | 104 | 330 | 4 | 228 | Ubiquitin carboxyl-terminal hydrolase isozyme L3 OS=Homo sapiens OX=9606 GN=UCHL3 PE=1 SV=1 |
|
| 8.79e-60 | 104 | 330 | 4 | 228 | Ubiquitin carboxyl-terminal hydrolase isozyme L3 OS=Mus musculus OX=10090 GN=Uchl3 PE=1 SV=2 |
|
| 1.20e-59 | 104 | 330 | 4 | 228 | Ubiquitin carboxyl-terminal hydrolase isozyme L3 OS=Bos taurus OX=9913 GN=UCHL3 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
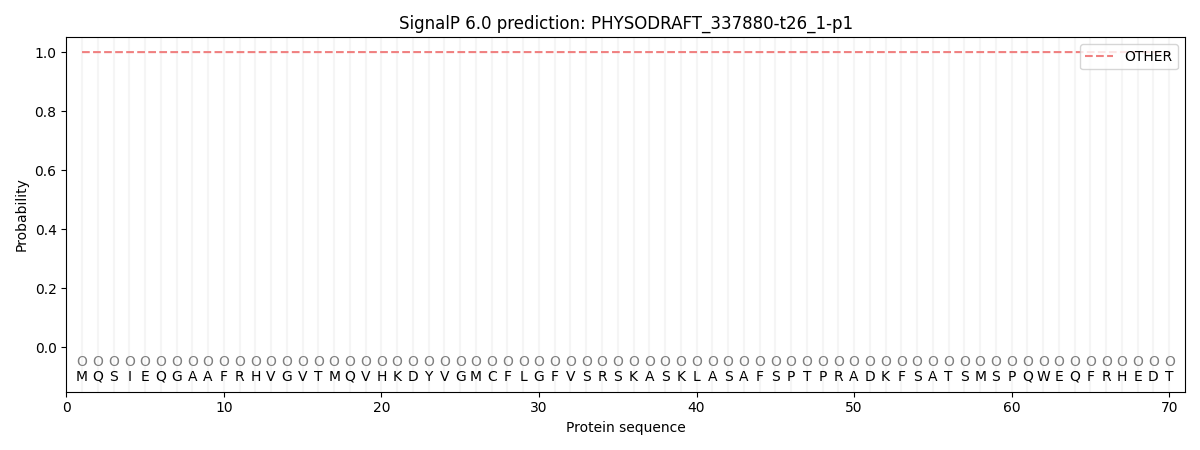
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000031 | 0.000000 |
