You are browsing environment: FUNGIDB
CAZyme Information: PHYBL_33968T0-p1
You are here: Home > Sequence: PHYBL_33968T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phycomyces blakesleeanus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Mucoromycota; Mucoromycetes; ; Phycomycetaceae; Phycomyces; Phycomyces blakesleeanus | |||||||||||
| CAZyme ID | PHYBL_33968T0-p1 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | Protein phosphatase, regulatory subunit PPP1R3C/D | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH15 | 186 | 589 | 7.8e-78 | 0.9501385041551247 |
| CBM21 | 27 | 125 | 2.6e-17 | 0.9719626168224299 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395586 | Glyco_hydro_15 | 5.41e-102 | 177 | 590 | 5 | 416 | Glycosyl hydrolases family 15. In higher organisms this family is represented by phosphorylase kinase subunits. |
| 397445 | CBM_21 | 1.18e-16 | 29 | 125 | 7 | 112 | Carbohydrate/starch-binding module (family 21). This family consists of several eukaryotic proteins that are thought to be involved in the regulation of glycogen metabolism. For instance, the mouse PTG protein has been shown to interact with glycogen synthase, phosphorylase kinase, phosphorylase a: these three enzymes have key roles in the regulation of glycogen metabolism. PTG also binds the catalytic subunit of protein phosphatase 1 (PP1C) and localizes it to glycogen. Subsets of similar interactions have been observed with several other members of this family, such as the yeast PIG1, PIG2, GAC1 and GIP2 proteins. While the precise function of these proteins is not known, they may serve a scaffold function, bringing together the key enzymes in glycogen metabolism. This family is a carbohydrate binding domain. |
| 225922 | SGA1 | 4.30e-16 | 175 | 590 | 249 | 601 | Glucoamylase (glucan-1,4-alpha-glucosidase), GH15 family [Carbohydrate transport and metabolism]. |
| 273702 | oligosac_amyl | 1.80e-10 | 328 | 584 | 401 | 604 | oligosaccharide amylase. The name of this type of amylase is based on the characterization of an glucoamylase family enzyme from Thermoactinomyces vulgaris. The T. vulgaris enzyme was expressed in E. coli and, like other glucoamylases, it releases beta-D-glucose from starch. However, unlike previously characterized glucoamylases, this T. vulgaris amylase hydrolyzes maltooligosaccharides (maltotetraose, maltose) more efficiently than starch (1), indicating this enzyme belongs to a class of glucoamylase-type enzymes with oligosaccharide-metabolizing activity. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.14e-238 | 22 | 595 | 26 | 600 | |
| 1.14e-238 | 22 | 595 | 26 | 600 | |
| 1.14e-238 | 22 | 595 | 26 | 600 | |
| 1.62e-238 | 22 | 595 | 26 | 600 | |
| 1.62e-238 | 22 | 595 | 26 | 600 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.77e-78 | 162 | 597 | 1 | 432 | Refined structure for the complex of acarbose with glucoamylase from Aspergillus awamori var. x100 to 2.4 angstroms resolution [Aspergillus awamori],1DOG_A REFINED STRUCTURE FOR THE COMPLEX OF 1-DEOXYNOJIRIMYCIN WITH GLUCOAMYLASE FROM (ASPERGILLUS AWAMORI) VAR. X100 TO 2.4 ANGSTROMS RESOLUTION [Aspergillus awamori],1GLM_A REFINED CRYSTAL STRUCTURES OF GLUCOAMYLASE FROM ASPERGILLUS AWAMORI VAR. X100 [Aspergillus awamori],3GLY_A REFINED CRYSTAL STRUCTURES OF GLUCOAMYLASE FROM ASPERGILLUS AWAMORI VAR. X100 [Aspergillus awamori] |
|
| 4.90e-78 | 162 | 597 | 1 | 432 | GLUCOAMYLASE-471 COMPLEXED WITH ACARBOSE [Aspergillus awamori] |
|
| 5.04e-78 | 162 | 597 | 1 | 432 | GLUCOAMYLASE-471 COMPLEXED WITH D-GLUCO-DIHYDROACARBOSE [Aspergillus awamori] |
|
| 1.96e-76 | 162 | 597 | 1 | 433 | Catalytic domain of glucoamylase from aspergillus niger complexed with tris and glycerol [Aspergillus niger] |
|
| 6.69e-75 | 162 | 597 | 1 | 433 | Structure of the catalytic domain of Aspergillus niger Glucoamylase [Aspergillus niger] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.70e-238 | 22 | 595 | 26 | 600 | Glucoamylase 1 OS=Rhizopus oryzae OX=64495 PE=1 SV=2 |
|
| 8.96e-146 | 164 | 595 | 34 | 447 | Glucoamylase amyD OS=Rhizopus delemar (strain RA 99-880 / ATCC MYA-4621 / FGSC 9543 / NRRL 43880) OX=246409 GN=amyD PE=1 SV=1 |
|
| 7.85e-83 | 29 | 596 | 34 | 611 | Glucoamylase OS=Blastobotrys adeninivorans OX=409370 GN=GAA PE=3 SV=1 |
|
| 2.02e-75 | 162 | 597 | 25 | 456 | Glucoamylase I OS=Aspergillus kawachii OX=1069201 GN=gaI PE=1 SV=1 |
|
| 1.40e-74 | 179 | 597 | 40 | 455 | Glucoamylase ARB_02327-1 OS=Schizophyllum commune (strain H4-8 / FGSC 9210) OX=578458 GN=SCHCODRAFT_57589 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
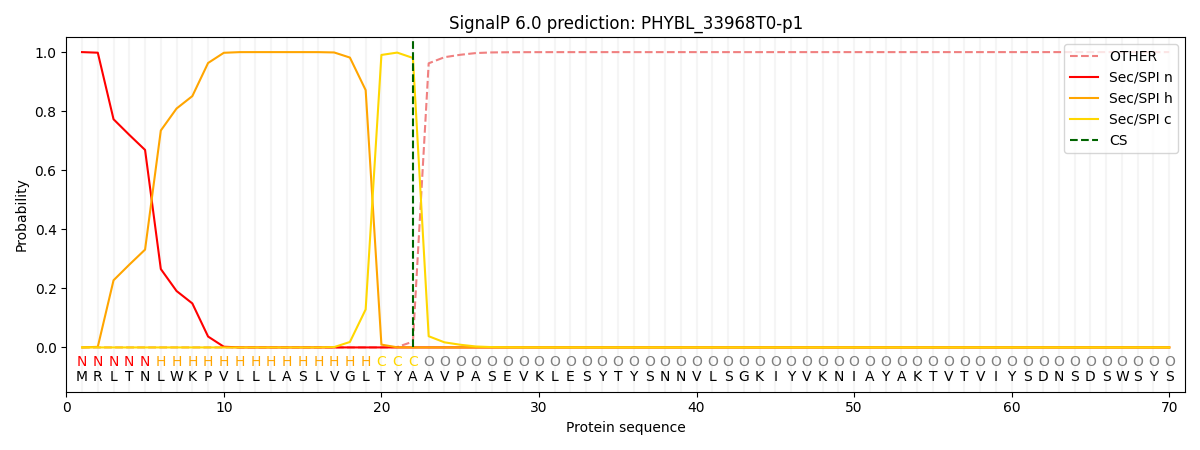
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000217 | 0.999742 | CS pos: 22-23. Pr: 0.9802 |
