You are browsing environment: FUNGIDB
CAZyme Information: PADG_07705-t30_1-p1
You are here: Home > Sequence: PADG_07705-t30_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Paracoccidioides brasiliensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; NA; Paracoccidioides; Paracoccidioides brasiliensis | |||||||||||
| CAZyme ID | PADG_07705-t30_1-p1 | |||||||||||
| CAZy Family | GT34 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH55 | 105 | 861 | 8.6e-248 | 0.9878378378378379 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 403800 | Pectate_lyase_3 | 7.99e-72 | 132 | 367 | 1 | 213 | Pectate lyase superfamily protein. This family of proteins possesses a beta helical structure like Pectate lyase. This family is most closely related to glycosyl hydrolase family 28. |
| 173787 | Peptidases_S8_S53 | 1.47e-21 | 1498 | 1748 | 1 | 226 | Peptidase domain in the S8 and S53 families. Members of the peptidases S8 (subtilisin and kexin) and S53 (sedolisin) family include endopeptidases and exopeptidases. The S8 family has an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. Serine acts as a nucleophile, aspartate as an electrophile, and histidine as a base. The S53 family contains a catalytic triad Glu/Asp/Ser with an additional acidic residue Asp in the oxyanion hole, similar to that of subtilisin. The serine residue here is the nucleophilic equivalent of the serine residue in the S8 family, while glutamic acid has the same role here as the histidine base. However, the aspartic acid residue that acts as an electrophile is quite different. In S53, it follows glutamic acid, while in S8 it precedes histidine. The stability of these enzymes may be enhanced by calcium; some members have been shown to bind up to 4 ions via binding sites with different affinity. There is a great diversity in the characteristics of their members: some contain disulfide bonds, some are intracellular while others are extracellular, some function at extreme temperatures, and others at high or low pH values. |
| 173790 | Peptidases_S8_PCSK9_ProteinaseK_like | 6.93e-18 | 1488 | 1766 | 17 | 250 | Peptidase S8 family domain in ProteinaseK-like proteins. The peptidase S8 or Subtilase clan of proteases have a Asp/His/Ser catalytic triad that is not homologous to trypsin. This CD contains several members of this clan including: PCSK9 (Proprotein convertase subtilisin/kexin type 9), Proteinase_K, Proteinase_T, and other subtilisin-like serine proteases. PCSK9 posttranslationally regulates hepatic low-density lipoprotein receptors (LDLRs) by binding to LDLRs on the cell surface, leading to their degradation. The binding site of PCSK9 has been localized to the epidermal growth factor-like repeat A (EGF-A) domain of the LDLR. Characterized Proteinases K are secreted endopeptidases with a high degree of sequence conservation. Proteinases K are not substrate-specific and function in a wide variety of species in different pathways. It can hydrolyze keratin and other proteins with subtilisin-like specificity. The number of calcium-binding motifs found in these differ. Proteinase T is a novel proteinase from the fungus Tritirachium album Limber. The amino acid sequence of proteinase T as deduced from the nucleotide sequence is about 56% identical to that of proteinase K. |
| 224322 | AprE | 2.15e-10 | 1489 | 1743 | 135 | 374 | Serine protease, subtilisin family [Posttranslational modification, protein turnover, chaperones]. |
| 395035 | Peptidase_S8 | 7.56e-09 | 1495 | 1751 | 1 | 258 | Subtilase family. Subtilases are a family of serine proteases. They appear to have independently and convergently evolved an Asp/Ser/His catalytic triad, like that found in the trypsin serine proteases (see pfam00089). Structure is an alpha/beta fold containing a 7-stranded parallel beta sheet, order 2314567. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 84 | 2114 | 9 | 2015 | |
| 0.0 | 42 | 1614 | 1 | 1547 | |
| 0.0 | 1 | 1189 | 1 | 1190 | |
| 0.0 | 42 | 1972 | 1 | 1991 | |
| 0.0 | 42 | 1972 | 1 | 1987 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.18e-178 | 107 | 859 | 2 | 751 | Chain A, Beta-1,3-glucanase [Thermochaetoides thermophila],5M60_A Chain A, Beta-1,3-glucanase [Thermochaetoides thermophila] |
|
| 2.95e-175 | 111 | 861 | 26 | 755 | Chain A, Glucan 1,3-beta-glucosidase [Phanerodontia chrysosporium],3EQN_B Chain B, Glucan 1,3-beta-glucosidase [Phanerodontia chrysosporium],3EQO_A Chain A, Glucan 1,3-beta-glucosidase [Phanerodontia chrysosporium],3EQO_B Chain B, Glucan 1,3-beta-glucosidase [Phanerodontia chrysosporium] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.97e-170 | 109 | 861 | 41 | 873 | Probable glucan endo-1,3-beta-glucosidase ARB_02077 OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_02077 PE=1 SV=1 |
|
| 2.36e-146 | 88 | 833 | 29 | 757 | Glucan 1,3-beta-glucosidase OS=Cochliobolus carbonum OX=5017 GN=EXG1 PE=1 SV=1 |
|
| 2.24e-48 | 104 | 867 | 30 | 756 | Glucan endo-1,3-beta-glucosidase BGN13.1 OS=Trichoderma harzianum OX=5544 GN=bgn13.1 PE=1 SV=1 |
|
| 6.41e-11 | 1488 | 1766 | 142 | 379 | Subtilisin-like protease 3 OS=Pseudogymnoascus destructans (strain ATCC MYA-4855 / 20631-21) OX=658429 GN=SP3 PE=1 SV=1 |
|
| 7.82e-09 | 1471 | 1749 | 125 | 368 | Subtilisin-like protease CPC735_013710 OS=Coccidioides posadasii (strain C735) OX=222929 GN=CPC735_013710 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
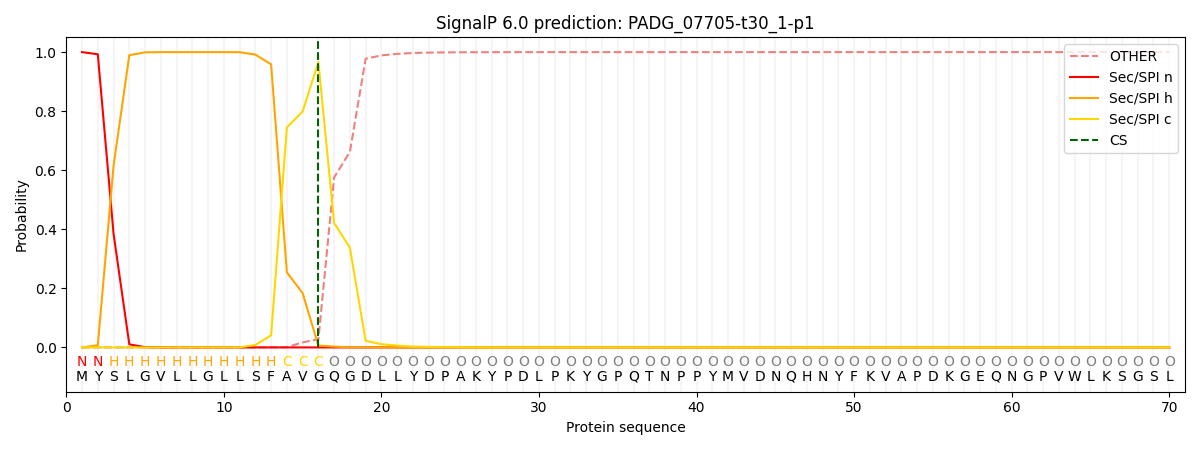
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000231 | 0.999729 | CS pos: 16-17. Pr: 0.9662 |
