You are browsing environment: FUNGIDB
CAZyme Information: OEJ89219.1
You are here: Home > Sequence: OEJ89219.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Hanseniaspora uvarum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Saccharomycetes; ; Saccharomycodaceae; Hanseniaspora; Hanseniaspora uvarum | |||||||||||
| CAZyme ID | OEJ89219.1 | |||||||||||
| CAZy Family | GT22 | |||||||||||
| CAZyme Description | Cytochrome c peroxidase, mitochondrial | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 1.11.1.5:2 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 74 | 310 | 7.9e-52 | 0.9176470588235294 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173825 | ascorbate_peroxidase | 2.83e-114 | 54 | 315 | 13 | 253 | Ascorbate peroxidases and cytochrome C peroxidases. Ascorbate peroxidases are a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Along with related catalase-peroxidases, ascorbate peroxidases belong to class I of the plant superfamily. Ascorbate peroxidases are found in the chloroplasts and/or cytosol of algae and plants, where they have been shown to control the concentration of lethal hydrogen peroxide molecules. The yeast cytochrome c peroxidase is a divergent member of the family; it forms a complex with cytochrome c to catalyze the reduction of hydrogen peroxide to water. |
| 223453 | KatG | 2.90e-84 | 41 | 316 | 56 | 421 | Catalase (peroxidase I) [Inorganic ion transport and metabolism]. |
| 178218 | PLN02608 | 1.67e-55 | 76 | 311 | 29 | 243 | L-ascorbate peroxidase |
| 178467 | PLN02879 | 2.70e-52 | 77 | 311 | 33 | 246 | L-ascorbate peroxidase |
| 173823 | plant_peroxidase_like | 5.81e-52 | 60 | 309 | 2 | 255 | Heme-dependent peroxidases similar to plant peroxidases. Along with animal peroxidases, these enzymes belong to a group of peroxidases containing a heme prosthetic group (ferriprotoporphyrin IX), which catalyzes a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. The plant peroxidase-like superfamily is found in all three kingdoms of life and carries out a variety of biosynthetic and degradative functions. Several sub-families can be identified. Class I includes intracellular peroxidases present in fungi, plants, archaea and bacteria, called catalase-peroxidases, that can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. Catalase-peroxidases are typically comprised of two homologous domains that probably arose via a single gene duplication event. Class II includes ligninase and other extracellular fungal peroxidases, while class III is comprised of classic extracellular plant peroxidases, like horseradish peroxidase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 5.54e-121 | 44 | 330 | 70 | 353 | |
| 1.11e-120 | 54 | 330 | 80 | 353 | |
| 1.37e-119 | 50 | 331 | 79 | 357 | |
| 1.37e-119 | 50 | 331 | 79 | 357 | |
| 1.37e-119 | 50 | 331 | 79 | 357 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.04e-117 | 54 | 331 | 19 | 294 | Chain A, Cytochrome c peroxidase [Saccharomyces cerevisiae],2JTI_A Chain A, Cytochrome c peroxidase, mitochondrial [Saccharomyces cerevisiae],6P43_A Yeast cytochrome c peroxidase in complex with iso-1 cytochrome c (Y48K) [Saccharomyces cerevisiae S288C],6P43_C Yeast cytochrome c peroxidase in complex with iso-1 cytochrome c (Y48K) [Saccharomyces cerevisiae S288C] |
|
| 8.61e-117 | 54 | 331 | 21 | 296 | X-RAY STRUCTURES OF RECOMBINANT YEAST CYTOCHROME C PEROXIDASE AND THREE HEME-CLEFT MUTANTS PREPARED BY SITE-DIRECTED MUTAGENESIS [Saccharomyces cerevisiae],1U74_A Chain A, cytochrome c peroxidase [Saccharomyces cerevisiae],1U74_C Chain C, cytochrome c peroxidase [Saccharomyces cerevisiae],1U75_A Chain A, cytochrome c peroxidase [Saccharomyces cerevisiae],1U75_C Chain C, cytochrome c peroxidase [Saccharomyces cerevisiae],2BCN_A Chain A, cytochrome c peroxidase [Saccharomyces cerevisiae],2BCN_C Chain C, cytochrome c peroxidase [Saccharomyces cerevisiae],2PCB_A Crystal Structure Of A Complex Between Electron Transfer Partners, Cytochrome C Peroxidase And Cytochrome C [Saccharomyces cerevisiae],2PCB_C Crystal Structure Of A Complex Between Electron Transfer Partners, Cytochrome C Peroxidase And Cytochrome C [Saccharomyces cerevisiae],2PCC_A Crystal Structure Of A Complex Between Electron Transfer Partners, Cytochrome C Peroxidase And Cytochrome C [Saccharomyces cerevisiae],2PCC_C Crystal Structure Of A Complex Between Electron Transfer Partners, Cytochrome C Peroxidase And Cytochrome C [Saccharomyces cerevisiae] |
|
| 8.91e-117 | 54 | 331 | 22 | 297 | THE ASP-HIS-FE TRIAD OF CYTOCHROME C PEROXIDASE CONTROLS THE REDUCTION POTENTIAL, ELECTRONIC STRUCTURE, AND COUPLING OF THE TRYPTOPHAN FREE-RADICAL TO THE HEME [Saccharomyces cerevisiae] |
|
| 2.07e-116 | 54 | 331 | 16 | 291 | ALTERING SUBSTRATE SPECIFICITY OF CYTOCHROME C PEROXIDASE TOWARDS A SMALL MOLECULAR SUBSTRATE PEROXIDASE BY SUBSTITUTING TYROSINE FOR PHE 202 [Saccharomyces cerevisiae] |
|
| 2.46e-116 | 54 | 331 | 21 | 296 | EFFECT OF ARGININE-48 REPLACEMENT ON THE REACTION BETWEEN CYTOCHROME C PEROXIDASE AND HYDROGEN PEROXIDE [Saccharomyces cerevisiae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.56e-123 | 45 | 331 | 63 | 346 | Cytochrome c peroxidase, mitochondrial OS=Kluyveromyces lactis (strain ATCC 8585 / CBS 2359 / DSM 70799 / NBRC 1267 / NRRL Y-1140 / WM37) OX=284590 GN=CCP1 PE=3 SV=1 |
|
| 2.43e-120 | 50 | 331 | 79 | 357 | Cytochrome c peroxidase, mitochondrial OS=Candida glabrata (strain ATCC 2001 / CBS 138 / JCM 3761 / NBRC 0622 / NRRL Y-65) OX=284593 GN=CAGL0K08184g PE=3 SV=1 |
|
| 3.19e-114 | 54 | 331 | 86 | 361 | Cytochrome c peroxidase, mitochondrial OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=CCP1 PE=1 SV=2 |
|
| 8.54e-88 | 9 | 330 | 29 | 359 | Cytochrome c peroxidase, mitochondrial OS=Debaryomyces hansenii (strain ATCC 36239 / CBS 767 / BCRC 21394 / JCM 1990 / NBRC 0083 / IGC 2968) OX=284592 GN=CCP1 PE=3 SV=1 |
|
| 5.38e-82 | 44 | 330 | 80 | 364 | Cytochrome c peroxidase, mitochondrial OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=CCP1 PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
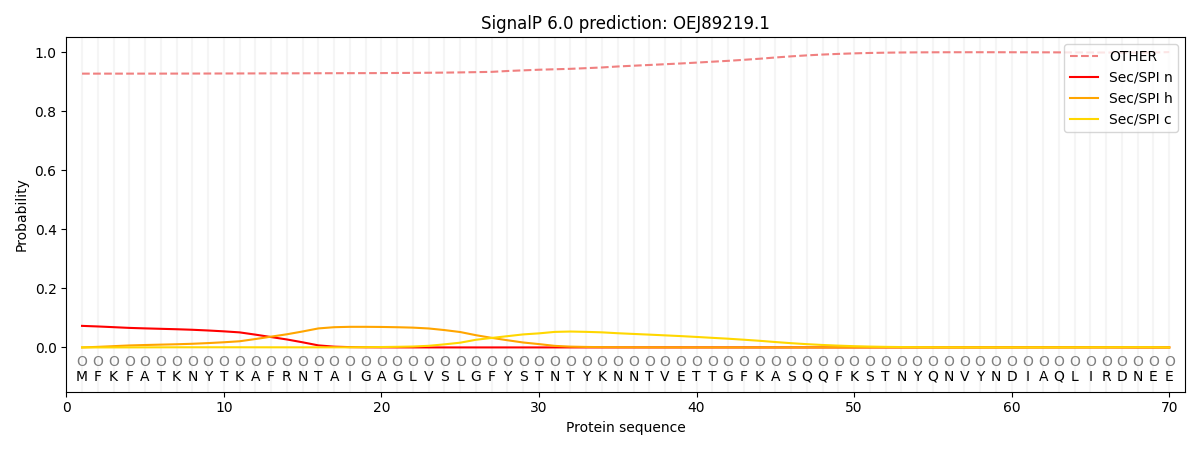
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.930664 | 0.069339 |
