You are browsing environment: FUNGIDB
CAZyme Information: OAG24007.1
You are here: Home > Sequence: OAG24007.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Alternaria alternata | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Pleosporaceae; Alternaria; Alternaria alternata | |||||||||||
| CAZyme ID | OAG24007.1 | |||||||||||
| CAZy Family | GH93 | |||||||||||
| CAZyme Description | Chitin synthase [Source:UniProtKB/TrEMBL;Acc:A0A177DWI4] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.16:26 | 2.4.1.16:26 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 1114 | 1276 | 1.7e-79 | 0.9938650306748467 |
| GT2 | 1254 | 1482 | 6.9e-21 | 0.4098671726755218 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 236223 | PRK08294 | 2.71e-120 | 1 | 585 | 45 | 603 | phenol 2-monooxygenase; Provisional |
| 396286 | Chitin_synth_1 | 4.51e-114 | 1114 | 1276 | 1 | 163 | Chitin synthase. This region is found commonly in chitin synthases classes I, II and III. Chitin a linear homopolymer of GlcNAc residues, it is an important component of the cell wall of fungi and is synthesized on the cytoplasmic surface of the cell membrane by membrane bound chitin synthases. |
| 133033 | Chitin_synth_C | 1.07e-91 | 1110 | 1430 | 1 | 244 | C-terminal domain of Chitin Synthase catalyzes the incorporation of GlcNAc from substrate UDP-GlcNAc into chitin. Chitin synthase, also called UDP-N-acetyl-D-glucosamine:chitin 4-beta-N-acetylglucosaminyltransferase, catalyzes the incorporation of GlcNAc from substrate UDP-GlcNAc into chitin, which is a linear homopolymer of GlcNAc residues formed by covalent beta-1,4 linkages. Chitin is an important component of the cell wall of fungi and bacteria and it is synthesized on the cytoplasmic surface of the cell membrane by membrane bound chitin synthases. Studies with fungi have revealed that most of them contain more than one chitin synthase gene. At least five subclasses of chitin synthases have been identified. |
| 400366 | Phe_hydrox_dim | 3.89e-58 | 404 | 582 | 1 | 166 | Phenol hydroxylase, C-terminal dimerization domain. Phenol hydroxylase acts a homodimer, to hydroxylates phenol to catechol or similar product. The enzyme is comprised of three domains. The first two domains from the active site. The third domain, this domain, is involved in forming the dimerization interface. The domain adopts a thioredoxin-like fold. |
| 396193 | FAD_binding_3 | 3.01e-47 | 1 | 368 | 14 | 348 | FAD binding domain. This domain is involved in FAD binding in a number of enzymes. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 715 | 1834 | 1 | 1122 | |
| 0.0 | 715 | 1834 | 95 | 1130 | |
| 0.0 | 885 | 1834 | 1 | 967 | |
| 0.0 | 927 | 1794 | 1 | 862 | |
| 0.0 | 726 | 1793 | 7 | 1095 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.89e-162 | 12 | 607 | 37 | 641 | Phenol hydroxylase from Trichosporon cutaneum [Cutaneotrichosporon cutaneum],1PN0_B Phenol hydroxylase from Trichosporon cutaneum [Cutaneotrichosporon cutaneum],1PN0_C Phenol hydroxylase from Trichosporon cutaneum [Cutaneotrichosporon cutaneum],1PN0_D Phenol hydroxylase from Trichosporon cutaneum [Cutaneotrichosporon cutaneum] |
|
| 2.77e-159 | 12 | 607 | 36 | 640 | Phenol Hydroxylase From Trichosporon Cutaneum [Cutaneotrichosporon cutaneum],1FOH_B Phenol Hydroxylase From Trichosporon Cutaneum [Cutaneotrichosporon cutaneum],1FOH_C Phenol Hydroxylase From Trichosporon Cutaneum [Cutaneotrichosporon cutaneum],1FOH_D Phenol Hydroxylase From Trichosporon Cutaneum [Cutaneotrichosporon cutaneum] |
|
| 2.81e-76 | 12 | 585 | 57 | 609 | Crystal structure of 3-hydroxybenzoate hydroxylase from Comamonas testosteroni, in complex with the substrate [Comamonas testosteroni],2DKI_A Crystal structure of 3-hydroxybenzoate hydroxylase from Comamonas testosteroni, under pressure of xenon gas (12 atm) [Comamonas testosteroni] |
|
| 2.73e-15 | 302 | 440 | 276 | 418 | Tracing the Evolution of Angucyclinone Monooxygenases: Structural Determinants for C-12b Hydroxylation and Substrate Inhibition in PgaE [Streptomyces sp. PGA64] |
|
| 2.73e-15 | 302 | 440 | 276 | 418 | Crystal structure of PgaE, an aromatic hydroxylase involved in angucycline biosynthesis [Streptomyces sp. PGA64] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 903 | 1771 | 79 | 975 | Chitin synthase 2 OS=Paracoccidioides brasiliensis OX=121759 GN=CHS2 PE=3 SV=2 |
|
| 0.0 | 868 | 1826 | 30 | 977 | Chitin synthase 1 OS=Exophiala dermatitidis OX=5970 GN=CHS1 PE=3 SV=2 |
|
| 0.0 | 859 | 1772 | 22 | 959 | Chitin synthase A OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=chsA PE=3 SV=2 |
|
| 0.0 | 909 | 1770 | 152 | 1060 | Chitin synthase 2 OS=Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) OX=367110 GN=chs-2 PE=3 SV=4 |
|
| 1.26e-282 | 1022 | 1759 | 89 | 837 | Chitin synthase 1 OS=Phycomyces blakesleeanus (strain ATCC 8743b / DSM 1359 / FGSC 10004 / NBRC 33097 / NRRL 1555) OX=763407 GN=chs1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000068 | 0.000000 |
TMHMM Annotations download full data without filtering help
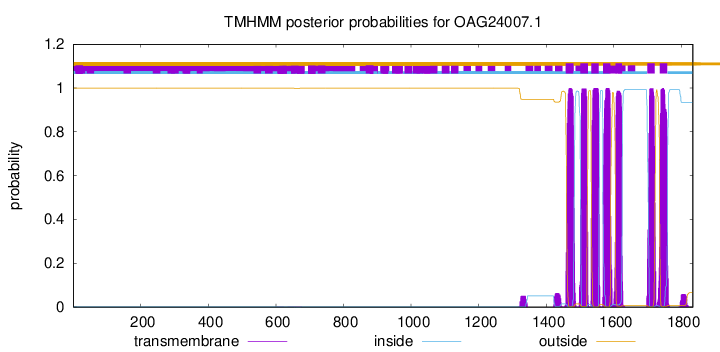
| Start | End |
|---|---|
| 1458 | 1480 |
| 1501 | 1523 |
| 1533 | 1555 |
| 1568 | 1590 |
| 1605 | 1624 |
| 1699 | 1721 |
| 1736 | 1758 |
