You are browsing environment: FUNGIDB
CAZyme Information: OAG16129.1
You are here: Home > Sequence: OAG16129.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Alternaria alternata | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Pleosporaceae; Alternaria; Alternaria alternata | |||||||||||
| CAZyme ID | OAG16129.1 | |||||||||||
| CAZy Family | AA9 | |||||||||||
| CAZyme Description | FAD-binding domain-containing protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 58 | 266 | 3.1e-47 | 0.45414847161572053 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 223354 | GlcD | 5.10e-09 | 60 | 499 | 23 | 449 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 215242 | PLN02441 | 1.30e-06 | 63 | 228 | 59 | 232 | cytokinin dehydrogenase |
| 396238 | FAD_binding_4 | 2.32e-06 | 69 | 206 | 1 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 178402 | PLN02805 | 0.002 | 69 | 229 | 134 | 299 | D-lactate dehydrogenase [cytochrome] |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.30e-15 | 67 | 235 | 46 | 214 | |
| 1.30e-15 | 67 | 235 | 46 | 214 | |
| 1.30e-15 | 67 | 235 | 46 | 214 | |
| 2.46e-15 | 56 | 233 | 49 | 228 | |
| 5.88e-15 | 69 | 233 | 61 | 226 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.05e-17 | 61 | 235 | 39 | 216 | Physcomitrella patens BBE-like 1 wild-type [Physcomitrium patens],6EO4_B Physcomitrella patens BBE-like 1 wild-type [Physcomitrium patens] |
|
| 6.05e-17 | 61 | 235 | 39 | 216 | Physcomitrella patens BBE-like 1 variant D396N [Physcomitrium patens],6EO5_B Physcomitrella patens BBE-like 1 variant D396N [Physcomitrium patens] |
|
| 2.03e-16 | 69 | 233 | 61 | 226 | Xylooligosaccharide oxidase from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],5L6F_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylobiose [Thermothelomyces thermophilus ATCC 42464],5L6G_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylose [Thermothelomyces thermophilus ATCC 42464] |
|
| 9.98e-16 | 35 | 269 | 15 | 243 | The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_B The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_C The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_D The crystal structure of EncM T139V mutant [Streptomyces maritimus] |
|
| 1.77e-15 | 35 | 269 | 15 | 243 | The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_B The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_C The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_D The crystal structure of EncM V135M mutant [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.38e-75 | 51 | 508 | 46 | 498 | Uncharacterized FAD-linked oxidoreductase ARB_02372 OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_02372 PE=1 SV=1 |
|
| 3.51e-54 | 25 | 508 | 39 | 512 | FAD-dependent monooxygenase drtC OS=Aspergillus calidoustus OX=454130 GN=drtC PE=1 SV=1 |
|
| 2.79e-47 | 31 | 508 | 9 | 475 | FAD-dependent monooxygenase sdcF OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=sdcF PE=1 SV=1 |
|
| 7.30e-45 | 59 | 506 | 69 | 513 | FAD-dependent monooxygenase tpcD OS=Cochliobolus heterostrophus (strain C5 / ATCC 48332 / race O) OX=701091 GN=tpcD PE=1 SV=1 |
|
| 3.00e-42 | 17 | 509 | 18 | 507 | FAD-dependent monooxygenase prx3 OS=Penicillium rubens (strain ATCC 28089 / DSM 1075 / NRRL 1951 / Wisconsin 54-1255) OX=500485 GN=prx3 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
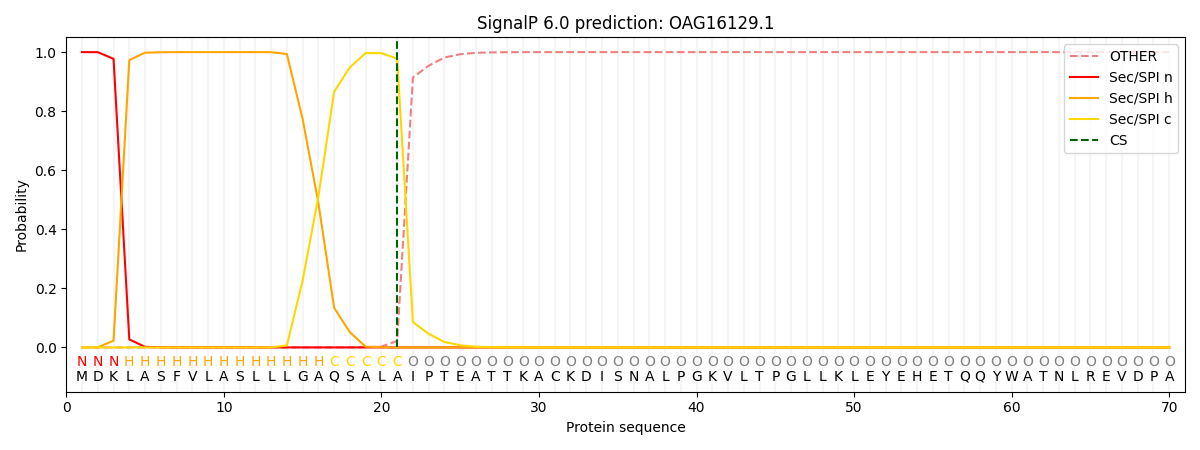
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000172 | 0.999798 | CS pos: 21-22. Pr: 0.9780 |
