You are browsing environment: FUNGIDB
CAZyme Information: OAG13667.1
You are here: Home > Sequence: OAG13667.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Alternaria alternata | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Pleosporaceae; Alternaria; Alternaria alternata | |||||||||||
| CAZyme ID | OAG13667.1 | |||||||||||
| CAZy Family | AA3 | |||||||||||
| CAZyme Description | Pkinase-domain-containing protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.96:2 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH18 | 374 | 643 | 6.7e-18 | 0.6959459459459459 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 270829 | STKc_CDK1_CdkB_like | 0.0 | 4 | 301 | 1 | 276 | Catalytic domain of Cyclin-Dependent protein Kinase 1-like Serine/Threonine Kinases and of Plant B-type Cyclin-Dependent protein Kinase. STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. This subfamily is composed of CDK, CDK2, and CDK3. CDK1 is also called Cell division control protein 2 (Cdc2) or p34 protein kinase, and is regulated by cyclins A, B, and E. The CDK1/cyclin A complex controls G2 phase entry and progression while the CDK1/cyclin B complex is critical for G2 to M phase transition. CDK2 is regulated by cyclin E or cyclin A. Upon activation by cyclin E, it phosphorylates the retinoblastoma (pRb) protein which activates E2F mediated transcription and allows cells to move into S phase. The CDK2/cyclin A complex plays a role in regulating DNA replication. Studies in knockout mice revealed that CDK1 can compensate for the loss of the cdk2 gene as it can also bind cyclin E and drive G1 to S phase transition. CDK3 is regulated by cyclin C and it phosphorylates pRB specifically during the G0/G1 transition. This phosphorylation is required for cells to exit G0 efficiently and enter the G1 phase. The plant-specific B-type CDKs are expressed from the late S to the M phase of the cell cycle. They are characterized by the cyclin binding motif PPT[A/T]LRE. They play a role in controlling mitosis and integrating developmental pathways, such as stomata and leaf development. CdkB has been shown to associate with both cyclin B, which controls G2/M transition, and cyclin D, which acts as a mediator in linking extracellular signals to the cell cycle. CDKs belong to a large family of STKs that are regulated by their cognate cyclins. Together, they are involved in the control of cell-cycle progression, transcription, and neuronal function. The CDK1 subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. |
| 270823 | STKc_CDK_like | 3.84e-174 | 4 | 301 | 1 | 275 | Catalytic domain of Cyclin-Dependent protein Kinase-like Serine/Threonine Kinases. STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. CDKs belong to a large family of STKs that are regulated by their cognate cyclins. Together, they are involved in the control of cell-cycle progression, transcription, and neuronal function. CDKs are partly regulated by their subcellular localization, which defines substrate phosphorylation and the resulting specific function. CDK1, CDK2, CDK4, and CDK6 have well-defined functions in the cell cycle, such as the regulation of the early G1 phase by CDK4 or CDK6, the G1/S phase transition by CDK2, or the entry of mitosis by CDK1. They also exhibit overlapping cyclin specificity and functions in certain conditions. Knockout mice with a single CDK deleted remain viable with specific phenotypes, showing that some CDKs can compensate for each other. For example, CDK4 can compensate for the loss of CDK6, however, double knockout mice with both CDK4 and CDK6 deleted die in utero. CDK8 and CDK9 are mainly involved in transcription while CDK5 is implicated in neuronal function. CDK7 plays essential roles in both the cell cycle as a CDK-Activating Kinase (CAK) and in transcription as a component of the general transcription factor TFIIH. The CDK-like subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. |
| 270844 | STKc_CDK2_3 | 2.99e-164 | 3 | 300 | 1 | 276 | Catalytic domain of the Serine/Threonine Kinases, Cyclin-Dependent protein Kinase 2 and 3. STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. CDK2 is regulated by cyclin E or cyclin A. Upon activation by cyclin E, it phosphorylates the retinoblastoma (pRb) protein which activates E2F mediated transcription and allows cells to move into S phase. The CDK2/cyclin A complex plays a role in regulating DNA replication. CDK2, together with CDK4, also regulates embryonic cell proliferation. Despite these important roles, mice deleted for the cdk2 gene are viable and normal except for being sterile. This may be due to compensation provided by CDK1 (also called Cdc2), which can also bind cyclin E and drive the G1 to S phase transition. CDK3 is regulated by cyclin C and it phosphorylates pRB specifically during the G0/G1 transition. This phosphorylation is required for cells to exit G0 efficiently and enter the G1 phase. CDKs belong to a large family of STKs that are regulated by their cognate cyclins. Together, they are involved in the control of cell-cycle progression, transcription, and neuronal function. The CDK2/3 subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. |
| 270845 | STKc_CDK1_euk | 1.57e-157 | 3 | 300 | 1 | 277 | Catalytic domain of the Serine/Threonine Kinase, Cyclin-Dependent protein Kinase 1 from higher eukaryotes. STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. CDK1 is also called Cell division control protein 2 (Cdc2) or p34 protein kinase, and is regulated by cyclins A, B, and E. The CDK1/cyclin A complex controls G2 phase entry and progression. CDK1/cyclin A2 has also been implicated as an important regulator of S phase events. The CDK1/cyclin B complex is critical for G2 to M phase transition. It induces mitosis by activating nuclear enzymes that regulate chromatin condensation, nuclear membrane degradation, mitosis-specific microtubule and cytoskeletal reorganization. CDK1 also associates with cyclin E and plays a role in the entry into S phase. CDK1 transcription is stable throughout the cell cycle but is modulated in some pathological conditions. It may play a role in regulating apoptosis under these conditions. In breast cancer cells, HER2 can mediate apoptosis by inactivating CDK1. Activation of CDK1 may contribute to HIV-1 induced apoptosis as well as neuronal apoptosis in neurodegenerative diseases. CDKs belong to a large family of STKs that are regulated by their cognate cyclins. Together, they are involved in the control of cell-cycle progression, transcription, and neuronal function. The CDK1 subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. |
| 177649 | PLN00009 | 1.01e-155 | 1 | 301 | 1 | 280 | cyclin-dependent kinase A; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 767 | 1 | 771 | |
| 3.45e-252 | 358 | 768 | 1 | 415 | |
| 2.10e-235 | 358 | 767 | 1 | 411 | |
| 4.43e-124 | 362 | 764 | 2 | 357 | |
| 1.16e-118 | 367 | 764 | 36 | 391 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.07e-129 | 1 | 302 | 2 | 281 | HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 2-Amino-6-(3'-methyl-2'-oxo)butoxypurine [Homo sapiens],1W8C_A CO-CRYSTAL STRUCTURE OF 6-CYCLOHEXYLMETHOXY-8-ISOPROPYL-9H-PURIN-2- YLAMINE AND MONOMERIC CDK2 [Homo sapiens],2R3I_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3J_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3K_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3L_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3M_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3N_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3O_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3P_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],2R3Q_A Crystal Structure of Cyclin-Dependent Kinase 2 with inhibitor [Homo sapiens],4NJ3_A Modulating the interaction between CDK2 and Cyclin A with a Quinoline-based inhibitor [Homo sapiens] |
|
| 5.20e-129 | 1 | 302 | 9 | 288 | CDK2 in complex with staurosporine [Homo sapiens],6Q3C_A CDK2 in complex with FragLite1 [Homo sapiens],6Q3F_A CDK2 in complex with FragLite2 [Homo sapiens],6Q4F_A CDK2 in complex with FragLite32 [Homo sapiens] |
|
| 7.82e-129 | 1 | 302 | 1 | 280 | Human Cyclin Dependent Kinase 2 Complexed With The Inhibitor Staurosporine [Homo sapiens],1BUH_A Crystal Structure Of The Human Cdk2 Kinase Complex With Cell Cycle-Regulatory Protein Ckshs1 [Homo sapiens],1CKP_A Human Cyclin Dependent Kinase 2 Complexed With The Inhibitor Purvalanol B [Homo sapiens],1DI8_A The Structure Of Cyclin-Dependent Kinase 2 (Cdk2) In Complex With 4-[3-Hydroxyanilino]-6,7-Dimethoxyquinazoline [Homo sapiens],1DM2_A HUMAN CYCLIN-DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR HYMENIALDISINE [Homo sapiens],1F5Q_A Crystal Structure Of Murine Gamma Herpesvirus Cyclin Complexed To Human Cyclin Dependent Kinase 2 [Homo sapiens],1F5Q_C Crystal Structure Of Murine Gamma Herpesvirus Cyclin Complexed To Human Cyclin Dependent Kinase 2 [Homo sapiens],1FIN_A Cyclin A-Cyclin-Dependent Kinase 2 Complex [Homo sapiens],1FIN_C Cyclin A-Cyclin-Dependent Kinase 2 Complex [Homo sapiens],1FVT_A THE STRUCTURE OF CYCLIN-DEPENDENT KINASE 2 (CDK2) IN COMPLEX WITH AN OXINDOLE INHIBITOR [Homo sapiens],1FVV_A The Structure Of Cdk2/cyclin A In Complex With An Oxindole Inhibitor [Homo sapiens],1FVV_C The Structure Of Cdk2/cyclin A In Complex With An Oxindole Inhibitor [Homo sapiens],1G5S_A CRYSTAL STRUCTURE OF HUMAN CYCLIN DEPENDENT KINASE 2 (CDK2) IN COMPLEX WITH THE INHIBITOR H717 [Homo sapiens],1GIH_A Human Cyclin Dependent Kinase 2 Complexed With The Cdk4 Inhibitor [Homo sapiens],1H0V_A Human cyclin dependent protein kinase 2 in complex with the inhibitor 2-Amino-6-[(R)-pyrrolidino-5'-yl]methoxypurine [Homo sapiens],1H0W_A Human cyclin dependent protein kinase 2 in complex with the inhibitor 2-Amino-6-[cyclohex-3-enyl]methoxypurine [Homo sapiens],1HCK_A Human Cyclin-Dependent Kinase 2 [Homo sapiens],1HCL_A Human Cyclin-Dependent Kinase 2 [Homo sapiens],1JSV_A The structure of cyclin-dependent kinase 2 (CDK2) in complex with 4-[(6-amino-4-pyrimidinyl)amino]benzenesulfonamide [Homo sapiens],1JVP_P Chain P, Cell division protein kinase 2 [Homo sapiens],1KE5_A CDK2 complexed with N-methyl-4-{[(2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]amino}benzenesulfonamide [Homo sapiens],1KE6_A CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH N-METHYL-{4-[2-(7-OXO-6,7-DIHYDRO-8H-[1,3]THIAZOLO[5,4-E]INDOL-8-YLIDENE)HYDRAZINO]PHENYL}METHANESULFONAMIDE [Homo sapiens],1KE7_A CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 3-{[(2,2-DIOXIDO-1,3-DIHYDRO-2-BENZOTHIEN-5-YL)AMINO]METHYLENE}-5-(1,3-OXAZOL-5-YL)-1,3-DIHYDRO-2H-INDOL-2-ONE [Homo sapiens],1KE8_A CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 4-{[(2-OXO-1,2-DIHYDRO-3H-INDOL-3-YLIDENE)METHYL]AMINO}-N-(1,3-THIAZOL-2-YL)BENZENESULFONAMIDE [Homo sapiens],1KE9_A CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 3-{[4-({[AMINO(IMINO)METHYL]AMINOSULFONYL)ANILINO]METHYLENE}-2-OXO-2,3-DIHYDRO-1H-INDOLE [Homo sapiens],1OKV_A Cyclin A binding groove inhibitor H-Arg-Arg-Leu-Ile-Phe-NH2 [Homo sapiens],1OKV_C Cyclin A binding groove inhibitor H-Arg-Arg-Leu-Ile-Phe-NH2 [Homo sapiens],1OKW_A Cyclin A binding groove inhibitor Ac-Arg-Arg-Leu-Asn-(m-Cl-Phe)-NH2 [Homo sapiens],1OKW_C Cyclin A binding groove inhibitor Ac-Arg-Arg-Leu-Asn-(m-Cl-Phe)-NH2 [Homo sapiens],1OL1_A Cyclin A binding groove inhibitor H-Cit-Cit-Leu-Ile-(p-F-Phe)-NH2 [Homo sapiens],1OL1_C Cyclin A binding groove inhibitor H-Cit-Cit-Leu-Ile-(p-F-Phe)-NH2 [Homo sapiens],1OL2_A Cyclin A binding groove inhibitor H-Arg-Arg-Leu-Asn-(p-F-Phe)-NH2 [Homo sapiens],1OL2_C Cyclin A binding groove inhibitor H-Arg-Arg-Leu-Asn-(p-F-Phe)-NH2 [Homo sapiens],1P2A_A The structure of cyclin dependent kinase 2 (CKD2) with a trisubstituted naphthostyril inhibitor [Homo sapiens],1PW2_A Apo Structure Of Human Cyclin-Dependent Kinase 2 [Homo sapiens],1PXI_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-(2,5-Dichloro-thiophen-3-yl)-pyrimidin-2-ylamine [Homo sapiens],1PXJ_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-ylamine [Homo sapiens],1PXK_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR N-[4-(2,4-Dimethyl-thiazol-5-yl)pyrimidin-2-yl]-N'-hydroxyiminoformamide [Homo sapiens],1PXL_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR [4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-amine [Homo sapiens],1PXM_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 3-[4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-ylamino]-phenol [Homo sapiens],1PXN_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-[4-(4-Methyl-2-methylamino-thiazol-5-yl)-pyrimidin-2-ylamino]-phenol [Homo sapiens],1PXO_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR [4-(2-Amino-4-methyl-thiazol-5-yl)-pyrimidin-2-yl]-(3-nitro-phenyl)-amine [Homo sapiens],1PXP_A HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR N-[4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-yl]-N',N'-dimethyl-benzene-1,4-diamine [Homo sapiens],1PYE_A Crystal structure of CDK2 with inhibitor [Homo sapiens],1R78_A CDK2 complex with a 4-alkynyl oxindole inhibitor [Homo sapiens],1URC_A Cyclin A binding groove inhibitor Ace-Arg-Lys-Leu-Phe-Gly [Homo sapiens],1URC_C Cyclin A binding groove inhibitor Ace-Arg-Lys-Leu-Phe-Gly [Homo sapiens],1VYZ_A Structure of CDK2 complexed with PNU-181227 [Homo sapiens],1W0X_C Crystal structure of human CDK2 in complex with the inhibitor olomoucine. [Homo sapiens],1WCC_A screening for fragment binding by X-ray crystallography [Homo sapiens],1Y8Y_A Crystal structure of human CDK2 complexed with a pyrazolo[1,5-a]pyrimidine inhibitor [Homo sapiens],1Y91_A Crystal structure of human CDK2 complexed with a pyrazolo[1,5-a]pyrimidine inhibitor [Homo sapiens],1YKR_A Crystal structure of cdk2 with an aminoimidazo pyridine inhibitor [Homo sapiens],2A0C_X Human CDK2 in complex with olomoucine II, a novel 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitor [Homo sapiens],2A4L_A Human cyclin-dependent kinase 2 in complex with roscovitine [Homo sapiens],2B52_A Human cyclin dependent kinase 2 (CDK2) complexed with DPH-042562 [Homo sapiens],2B53_A Human cyclin dependent kinase 2 (CDK2) complexed with DIN-234325 [Homo sapiens],2B54_A Human cyclin dependent kinase 2 (CKD2)complexed with DIN-232305 [Homo sapiens],2B55_A Human cyclin dependent kinase 2 (cdk2) complexed with indenopyraxole DIN-101312 [Homo sapiens],2BHE_A HUMAN CYCLIN DEPENDENT PROTEIN KINASE 2 IN COMPLEX WITH THE INHIBITOR 5-BROMO-INDIRUBINE [Homo sapiens],2BHH_A Human Cyclin Dependent Protein Kinase 2 In Complex With The Inhibitor 4-Hydroxypiperindinesulfonyl-Indirubine [Homo sapiens],2BTR_A STRUCTURE OF CDK2 COMPLEXED WITH PNU-198873 [Homo sapiens],2BTS_A STRUCTURE OF CDK2 COMPLEXED WITH PNU-230032 [Homo sapiens],2C5N_A Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5N_C Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5O_A Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5O_C Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5V_A Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5V_C Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5X_A Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5X_C Differential Binding Of Inhibitors To Active And Inactive Cdk2 Provides Insights For Drug Design [Homo sapiens],2C5Y_A DIFFERENTIAL BINDING OF INHIBITORS TO ACTIVE AND INACTIVE CDK2 PROVIDES INSIGHTS FOR DRUG DESIGN [Homo sapiens],2C68_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C69_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C6I_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C6K_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C6L_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C6M_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2C6O_A Crystal structure of the human CDK2 complexed with the triazolopyrimidine inhibitor [Homo sapiens],2CLX_A 4-Arylazo-3,5-diamino-1H-pyrazole CDK Inhibitors: SAR Study, Crystal Structure in Complex with CDK2, Selectivity, and Cellular Effects [Homo sapiens],2DUV_A Structure of CDK2 with a 3-hydroxychromones [Homo sapiens],2EXM_A Human CDK2 in complex with isopentenyladenine [Homo sapiens],2FVD_A Cyclin Dependent Kinase 2 (CDK2) with diaminopyrimidine inhibitor [Homo sapiens],2I40_A Cdk2/Cyclin A complexed with a thiophene carboxamide inhibitor [Homo sapiens],2I40_C Cdk2/Cyclin A complexed with a thiophene carboxamide inhibitor [Homo sapiens],2J9M_A Crystal Structure of CDK2 in complex with Macrocyclic Aminopyrimidine [Homo sapiens],2R64_A Crystal structure of a 3-aminoindazole compound with CDK2 [Homo sapiens],2UUE_A REPLACE: A strategy for Iterative Design of Cyclin Binding Groove Inhibitors [Homo sapiens],2UUE_C REPLACE: A strategy for Iterative Design of Cyclin Binding Groove Inhibitors [Homo sapiens],2UZN_A Crystal structure of human CDK2 complexed with a thiazolidinone inhibitor [Homo sapiens],2UZO_A Crystal structure of human CDK2 complexed with a thiazolidinone inhibitor [Homo sapiens],2V0D_A Crystal structure of human CDK2 complexed with a thiazolidinone inhibitor [Homo sapiens],2V22_A REPLACE: A strategy for Iterative Design of Cyclin Binding Groove Inhibitors [Homo sapiens],2V22_C REPLACE: A strategy for Iterative Design of Cyclin Binding Groove Inhibitors [Homo sapiens],2VTA_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTH_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design [Homo sapiens],2VTI_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTJ_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design [Homo sapiens],2VTL_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design [Homo sapiens],2VTM_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTN_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTO_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTP_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTQ_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTR_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design [Homo sapiens],2VTS_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VTT_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2VU3_A Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H- pyrazole-3-carboxamide (AT7519), a Novel Cyclin Dependent Kinase Inhibitor Using Fragment-Based X-Ray Crystallography and Structure Based Drug Design. [Homo sapiens],2W05_A Structure of CDK2 in complex with an imidazolyl pyrimidine, compound 5b [Homo sapiens],2W1H_A Fragment-Based Discovery of the Pyrazol-4-yl urea (AT9283), a Multi- targeted Kinase Inhibitor with Potent Aurora Kinase Activity [Homo sapiens],2WEV_A Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2WEV_C Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2WFY_A Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2WFY_C Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2WHB_A Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2WHB_C Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2X1N_A Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2X1N_C Truncation and Optimisation of Peptide Inhibitors of CDK2, Cyclin A Through Structure Guided Design [Homo sapiens],2XMY_A Discovery and Characterisation of 2-Anilino-4-(thiazol-5-yl) pyrimidine Transcriptional CDK Inhibitors as Anticancer Agents [Homo sapiens],2XNB_A Discovery and Characterisation of 2-Anilino-4-(thiazol-5-yl) pyrimidine Transcriptional CDK Inhibitors as Anticancer Agents [Homo sapiens],3EID_A CDK2/CyclinA complexed with a pyrazolopyridazine inhibitor [Homo sapiens],3EID_C CDK2/CyclinA complexed with a pyrazolopyridazine inhibitor [Homo sapiens],3EJ1_A CDK2/CyclinA complexed with a pyrazolopyridazine inhibitor [Homo sapiens],3EJ1_C CDK2/CyclinA complexed with a pyrazolopyridazine inhibitor [Homo sapiens],3EOC_A Cdk2/CyclinA complexed with a imidazo triazin-2-amine [Homo sapiens],3EOC_C Cdk2/CyclinA complexed with a imidazo triazin-2-amine [Homo sapiens],3F5X_A CDK-2-Cyclin complex with indazole inhibitor 9 bound at its active site [Homo sapiens],3F5X_C CDK-2-Cyclin complex with indazole inhibitor 9 bound at its active site [Homo sapiens],3FZ1_A Crystal structure of a benzthiophene inhibitor bound to human Cyclin-dependent Kinase-2 (CDK-2) [Homo sapiens],3LE6_A The structure of cyclin dependent kinase 2 (CKD2) with a pyrazolobenzodiazepine inhibitor [Homo sapiens],3LFN_A Crystal structure of CDK2 with SAR57, an aminoindazole type inhibitor [Homo sapiens],3LFQ_A Crystal structure of CDK2 with SAR60, an aminoindazole type inhibitor [Homo sapiens],3LFS_A Crystal structure of CDK2 with SAR37, an aminoindazole type inhibitor [Homo sapiens],3NS9_A Crystal structure of CDK2 in complex with inhibitor BS-194 [Homo sapiens],3S2P_A Crystal structure of CDK2 with a 2-aminopyrimidine compound [Homo sapiens],3TI1_A CDK2 in complex with SUNITINIB [Homo sapiens],3TIY_A CDK2 in complex with NSC 35676 [Homo sapiens],3TIZ_A CDK2 in complex with NSC 111848 [Homo sapiens],3ULI_A Human Cyclin Dependent Kinase 2 (CDK2) bound to azabenzimidazole derivative [Homo sapiens],3UNJ_A CDK2 in complex with inhibitor YL1-038-31 [Homo sapiens],3UNK_A CDK2 in complex with inhibitor YL5-083 [Homo sapiens],3WBL_A Crystal structure of CDK2 in complex with pyrazolopyrimidine inhibitor [Homo sapiens],4BGH_A Crystal Structure of CDK2 in complex with pan-CDK Inhibitor [Homo sapiens],4D1X_A CDK2 in complex with Luciferin [Homo sapiens],4D1Z_A CDK2 in complex with a Luciferin derivate [Homo sapiens],4FX3_A Crystal Structure of the CDK2/Cyclin A complex with oxindole inhibitor [Homo sapiens],4FX3_C Crystal Structure of the CDK2/Cyclin A complex with oxindole inhibitor [Homo sapiens],4KD1_A CDK2 in complex with Dinaciclib [Homo sapiens],4LYN_A Crystal structure of cyclin-dependent kinase 2 (cdk2-wt) complex with (2s)-n-(5-(((5-tert-butyl-1,3-oxazol-2-yl)methyl)sulfanyl)-1,3-thiazol-2-yl)-2-phenylpropanamide [Homo sapiens],5AND_A Crystal structure of CDK2 in complex with 2-imidazol-1-yl-1H- benzimidazole processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANE_A Crystal structure of CDK2 in complex with 6-methoxy-7H-purine processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANG_A Crystal structure of CDK2 in complex with 7-hydroxy-4-(morpholinomethyl)chromen-2-one processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANI_A Crystal structure of CDK2 in complex with 6-chloro-7H-purine processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANJ_A Crystal structure of CDK2 in complex with N-(9H-purin-6-yl)thiophene- 2-carboxamide processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANK_A Crystal structure of CDK2 in complex with 2,4,6-trioxo-1-phenyl- hexahydropyrimidine-5-carboxamide processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5ANO_A Crystal structure of CDK2 processed with the CrystalDirect automated mounting and cryo-cooling technology [Homo sapiens],5D1J_A CRYSTAL STRUCTURE OF CYCLIN-DEPENDENT KINASE 2 (CDK2-WT) COMPLEX WITH N-[5-[[[5-(1,1-DIMETHYLETHYL)-2-OXAZOLYL] METHYL]THIO]-2-THIAZOLYL]-4-PIPERIDINECARBOXAMIDE (BMS-387032) [Homo sapiens],5FP5_A Chain A, CYCLIN-DEPENDENT KINASE 2 [Homo sapiens],5FP6_A Chain A, CYCLIN-DEPENDENT KINASE 2 [Homo sapiens],5IEV_A Crystal structure of BAY 1000394 (Roniciclib) bound to CDK2 [Homo sapiens],5IEX_A Crystal structure of (R,S)-S-{4-[(5-Bromo-4-{[(2R,3R)-2-hydroxy-1-methylpropyl]oxy}- pyrimidin-2-yl)amino]phenyl}-S-cyclopropylsulfoximide bound to CDK2 [Homo sapiens],5IEY_A Crystal structure of a CDK inhibitor bound to CDK2 [Homo sapiens],5IF1_A Crystal structure apo CDK2/cyclin A [Homo sapiens],5IF1_C Crystal structure apo CDK2/cyclin A [Homo sapiens],5JQ5_A Crystal structure of CDK2 in complex with inhibitor ICEC0942 [Homo sapiens],5JQ8_A Crystal structure of CDK2 in complex with inhibitor ICEC0943 [Homo sapiens],6P3W_A Crystal structure of the Cyclin A-CDK2-ORC1 complex [Homo sapiens],6P3W_C Crystal structure of the Cyclin A-CDK2-ORC1 complex [Homo sapiens],7M2F_A Chain A, Cyclin-dependent kinase 2 [Homo sapiens] |
|
| 8.10e-129 | 1 | 302 | 2 | 281 | Crystal Structure of CDK2 in complex with compound 22 [Homo sapiens] |
|
| 8.10e-129 | 1 | 302 | 2 | 281 | Imidazopyridines: a potent and selective class of Cyclin-dependent Kinase inhibitors identified through Structure-based hybridisation [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.12e-180 | 1 | 304 | 1 | 302 | Cyclin-dependent kinase 1 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=nimX PE=2 SV=1 |
|
| 2.14e-178 | 1 | 304 | 1 | 303 | Cyclin-dependent kinase 1 OS=Ajellomyces capsulatus OX=5037 GN=CDC2 PE=3 SV=1 |
|
| 1.43e-149 | 1 | 304 | 5 | 291 | Cyclin-dependent kinase 1 OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=CDC28 PE=1 SV=1 |
|
| 1.79e-147 | 1 | 304 | 4 | 288 | Cyclin-dependent kinase 1 OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=CDC28 PE=1 SV=1 |
|
| 1.12e-133 | 1 | 302 | 1 | 287 | Cyclin-dependent kinase 1 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=cdc2 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
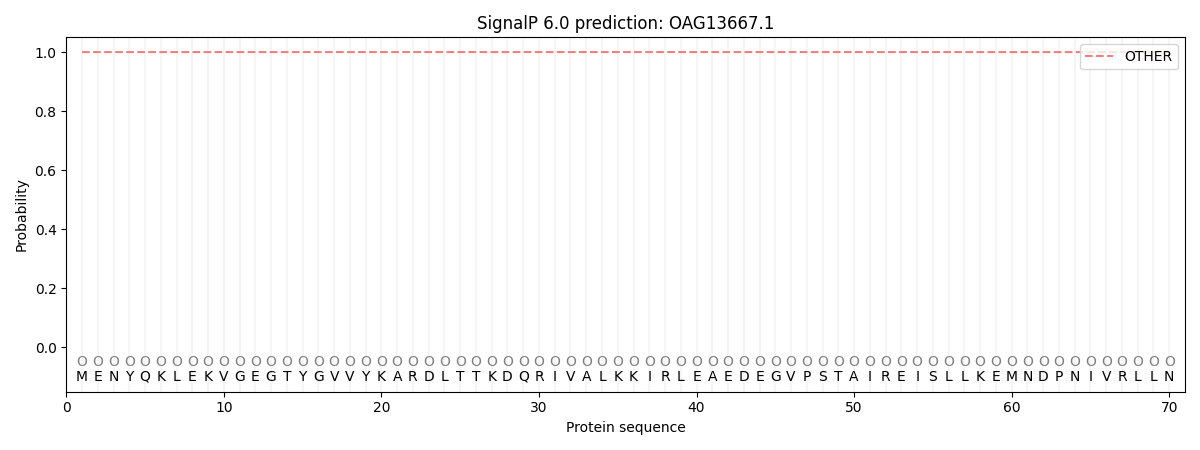
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000083 | 0.000000 |
