You are browsing environment: FUNGIDB
CAZyme Information: KNZ43953.1
You are here: Home > Sequence: KNZ43953.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
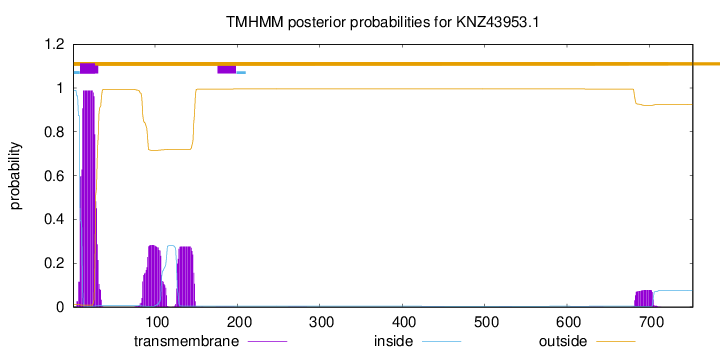
TMHMM annotations
Basic Information help
| Species | Puccinia sorghi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Pucciniomycetes; ; Pucciniaceae; Puccinia; Puccinia sorghi | |||||||||||
| CAZyme ID | KNZ43953.1 | |||||||||||
| CAZy Family | AA1 | |||||||||||
| CAZyme Description | uncharacterized protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 291 | 699 | 8.4e-26 | 0.5921787709497207 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 259950 | CuRO_2_Diphenol_Ox | 6.44e-63 | 257 | 416 | 11 | 152 | The second cupredoxin domain of fungal laccase, diphenol oxidase. Diphenol oxidase belongs to the laccase family. It catalyzes the initial steps in melanin biosynthesis from diphenols. Melanin is one of the virulence factors of infectious fungi. In the pathogenesis of C. neoformans, melanin pigments have been shown to protect the fungal cells from oxidative and microbicidal activities of host defense systems. Laccase is a blue multi-copper enzyme that catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism. Laccase is a multicopper oxidase (MCO) composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 2 of 3-domain MCOs has lost the ability to bind copper. |
| 259971 | CuRO_3_Diphenol_Ox | 1.46e-60 | 513 | 705 | 1 | 158 | The third cupredoxin domain of fungal laccase, diphenol oxidase. Diphenol oxidase belongs to the laccase family. It catalyzes the initial steps in melanin biosynthesis from diphenols. Melanin is one of the virulence factors of infectious fungi. In the pathogenesis of C. neoformans, melanin pigments have been shown to protect the fungal cells from oxidative and microbicidal activities of host defense systems. Laccase is a blue multicopper oxidase (MCO) which catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 3 of 3-domain MCOs contains the Type 1 (T1) copper binding site and part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259926 | CuRO_1_Diphenol_Ox | 1.37e-31 | 49 | 190 | 1 | 98 | The first cupredoxin domain of fungal laccase, diphenol oxidase. Diphenol oxidase belongs to the laccase family. It catalyzes the initial steps in melanin biosynthesis from diphenols. Melanin is one of the virulence factors of infectious fungi. In the pathogenesis of C. neoformans, melanin pigments have been shown to protect the fungal cells from oxidative and microbicidal activities of host defense systems. Laccase is a blue multicopper oxidase (MCO) which catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 259953 | CuRO_2_MCO_like_1 | 1.99e-30 | 299 | 411 | 33 | 144 | The second cupredoxin domain of uncharacterized multicopper oxidase. Multicopper Oxidases (MCOs) are multi-domain enzymes that are able to couple oxidation of substrates with reduction of dioxygen to water. MCOs oxidise their substrate by accepting electrons at a mononuclear copper centre and transferring them to a trinuclear copper centre which binds a dioxygen. The dioxygen, following the transfer of four electrons, is reduced to two molecules of water. These MCOs are capable of oxidizing a vast range of substrates, varying from aromatic to inorganic compounds such as metals. This family of MCOs is composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 2 of 3-domain MCOs has lost the ability to bind copper. |
| 259869 | CuRO_1_LCC_like | 1.02e-24 | 49 | 193 | 1 | 101 | Cupredoxin domain 1 of laccase-like multicopper oxidases; including laccase, CueO, spore coat protein A, ascorbate oxidase and similar proteins. Laccase-like multicopper oxidases (MCOs) in this family contain three cupredoxin domains. They are able to couple oxidation of substrates with reduction of dioxygen to water. MCOs are capable of oxidizing a vast range of substrates, varying from aromatic to inorganic compounds such as metals. Although the members of this family have diverse functions, majority of them have three cupredoxin domain repeats. The copper ions are bound in several sites; Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. Also included in this family are cupredoxin domains 1, 3, and 5 of the 6-domain MCO ceruloplasmin and similar proteins. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.44e-116 | 32 | 743 | 51 | 610 | |
| 3.42e-116 | 32 | 743 | 51 | 610 | |
| 3.76e-112 | 40 | 743 | 39 | 578 | |
| 3.76e-112 | 40 | 743 | 39 | 578 | |
| 9.26e-109 | 40 | 743 | 39 | 578 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.35e-29 | 58 | 730 | 7 | 495 | T2-depleted laccase from Coriolopsis caperata soaked with CuCl [Coriolopsis caperata],4JHV_A T2-depleted laccase from Coriolopsis caperata [Coriolopsis caperata] |
|
| 3.60e-28 | 57 | 730 | 6 | 495 | PM1 mutant, 7D5 [Aspergillus oryzae],6H5Y_B PM1 mutant, 7D5 [Aspergillus oryzae] |
|
| 2.08e-27 | 57 | 730 | 6 | 495 | Chain A, CORIOLOPSIS GALLICA LACCASE [Coriolopsis gallica] |
|
| 3.72e-27 | 57 | 730 | 6 | 495 | Crystal Structure Of Laccase From Basidiomycete Pm1 (cect 2971) [basidiomycete PM1],5ANH_B Crystal Structure Of Laccase From Basidiomycete Pm1 (cect 2971) [basidiomycete PM1],5ANH_C Crystal Structure Of Laccase From Basidiomycete Pm1 (cect 2971) [basidiomycete PM1] |
|
| 9.08e-27 | 57 | 723 | 6 | 487 | Chain A, Laccase [Lentinus tigrinus],2QT6_B Chain B, Laccase [Lentinus tigrinus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.49e-81 | 25 | 745 | 38 | 595 | Laccase-1 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC1 PE=1 SV=1 |
|
| 1.47e-79 | 25 | 743 | 38 | 593 | Laccase-2 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC2 PE=3 SV=2 |
|
| 1.11e-78 | 25 | 742 | 38 | 594 | Laccase-1 OS=Cryptococcus neoformans var. neoformans serotype D (strain B-3501A) OX=283643 GN=LAC1 PE=1 SV=1 |
|
| 5.99e-28 | 59 | 723 | 32 | 520 | Laccase-2 OS=Fomitopsis pinicola (strain FP-58527) OX=743788 GN=LCC2 PE=1 SV=1 |
|
| 9.99e-27 | 57 | 730 | 27 | 519 | Laccase-1 OS=Trametes villosa OX=47662 GN=LCC1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
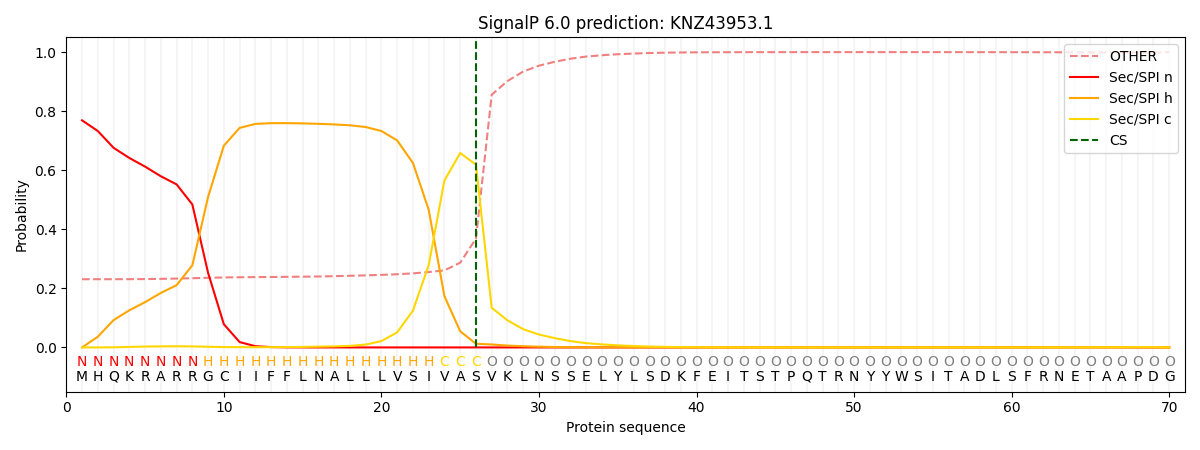
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.240774 | 0.759190 | CS pos: 26-27. Pr: 0.6189 |