You are browsing environment: FUNGIDB
CAZyme Information: KIW09927.1
You are here: Home > Sequence: KIW09927.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Exophiala spinifera | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Herpotrichiellaceae; Exophiala; Exophiala spinifera | |||||||||||
| CAZyme ID | KIW09927.1 | |||||||||||
| CAZy Family | AA1 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH64 | 161 | 534 | 3.4e-90 | 0.9591280653950953 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 185759 | GH64-GluB-like | 3.93e-142 | 156 | 534 | 1 | 368 | glycoside hydrolase family 64: beta-1,3-glucanase B (GluB)-like. This subfamily is represented by GluB, beta-1,3-glucanase B , from Lysobacter enzymogenes Strain N4-7 and related bacterial and ascomycete proteins. GluB is a member of the glycoside hydrolase family 64 (GH64) involved in the cleavage of long-chain polysaccharide beta-1,3-glucans, into specific pentasaccharide oligomers. Among bacteria, many beta-1,3-glucanases are implicated in fungal cell wall degradation. GluB possesses the conserved Glu and Asp residues required to cleave substrate beta-1,3-glucans. Recombinant GluB demonstrated higher relative activity toward the branched-chain beta-1,3 glucan substrate zymosan A than toward linear beta-1,3 glucan substrates. Based on the structure of laminaripentaose-producing, beta-1,3-glucanase (LPHase) of Streptomyces matensis, which belongs to the same family as GluB but to a different subfamily, this cd is a two-domain model. Sometimes these two domains are found associated with other domains such as in the Catenulispora acidiphila DSM 44928 carbohydrate binding family 6 protein in which they are positioned N-terminal of a carbohydrate binding module, family 6 (CBM_6) domain. |
| 406796 | Glyco_hydro_64 | 3.19e-135 | 154 | 534 | 1 | 370 | Beta-1,3-glucanase. Family 64 glycoside hydrolases have beta-1,3-glucanase activity. |
| 185755 | GH64-LPHase-like | 7.31e-65 | 156 | 534 | 1 | 352 | glycoside hydrolase family 64: laminaripentaose-producing, beta-1,3-glucanase (LPHase)-like. This subfamily is represented by the laminaripentaose-producing, beta-1,3-glucanase (LPHase) of Streptomyces matensis and related bacterial and ascomycete proteins. LPHase is a member of glycoside hydrolase family 64 (GH64), it is an inverting enzyme involved in the cleavage of long-chain polysaccharide beta-1,3-glucans, into specific pentasaccharide oligomers. LPHase is a two-domain crescent fold structure: one domain is composed of 10 beta-strands, eight coming from the N-terminus of the protein and two from the C-terminal region, and the protein has a second inserted domain; this cd includes both domains. This protein has an electronegative, substrate-binding cleft, and conserved Glu and Asp residues involved in the cleavage of the beta-1,3-glucan, laminarin, a plant and fungal cell wall component. Among bacteria, many beta-1,3-glucanases are implicated in fungal cell wall degradation. Also included in this family is GluB , the beta-1,3-glucanase B from Lysobacter enzymogenes Strain N4-7. Recombinant GluB demonstrated higher relative activity toward the branched-chain beta-1,3 glucan substrate zymosan A than toward linear beta-1,3 glucan substrates. Sometimes these two domains are found associated with other domains such as in the Catenulispora acidiphila DSM 44928 carbohydrate binding family 6 protein in which they are positioned N-terminal of a carbohydrate binding module, family 6 (CBM_6) domain. In the Cellulosimicrobium cellulans, glucan endo-1,3-beta-glucosidase, they are positioned N-terminal of a RICIN, carbohydrate-binding domain. |
| 185753 | GH64-like | 2.71e-30 | 168 | 535 | 15 | 319 | glycosyl hydrolase 64 family. This family is represented by the laminaripentaose-producing, beta-1,3-glucanase (LPHase) of Streptomyces matensis and related bacterial and ascomycete proteins. LPHase is a member of glycoside hydrolase family 64 (GH64), it is an inverting enzyme involved in the cleavage of long-chain polysaccharide beta-1,3-glucans, into specific pentasaccharide oligomers. LPHase is a two-domain crescent fold structure: one domain is composed of 10 beta-strands, eight coming from the N-terminus of the protein and two from the C-terminal region, and the protein has a second inserted domain; this cd includes both domains. This protein has an electronegative, substrate-binding cleft, and conserved Glu and Asp residues involved in the cleavage of the beta-1,3-glucan, laminarin, a plant and fungal cell wall component. Among bacteria, many beta-1,3-glucanases are implicated in fungal cell wall degradation. Also included in this family is GluB , the beta-1,3-glucanase B from Lysobacter enzymogenes Strain N4-7. Recombinant GluB demonstrated higher relative activity toward the branched-chain beta-1,3 glucan substrate zymosan A than toward linear beta-1,3 glucan substrates. Sometimes these two domains are found associated with other domains such as in the Catenulispora acidiphila DSM 44928 carbohydrate binding family 6 protein in which they are positioned N-terminal of a carbohydrate binding module, family 6 (CBM_6) domain. In the Cellulosimicrobium cellulans, glucan endo-1,3-beta-glucosidase, they are positioned N-terminal of a RICIN, carbohydrate-binding domain, and in the Salinispora tropica CNB-440, coagulation factor 5/8 C-terminal domain (FA58C) protein, they are positioned C-terminal of two FA58C domains which are proposed to function as cell surface-attached, carbohydrate-binding domain. This FA58C-containing protein has an internal peptide deletion (of approx. 44 residues) in the LPHase domain II. |
| 223730 | Aes | 1.48e-23 | 677 | 891 | 113 | 312 | Acetyl esterase/lipase [Lipid transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.79e-101 | 150 | 534 | 62 | 438 | |
| 1.32e-97 | 167 | 535 | 91 | 450 | |
| 1.64e-95 | 156 | 539 | 73 | 449 | |
| 3.70e-94 | 156 | 534 | 157 | 529 | |
| 4.54e-94 | 153 | 534 | 70 | 443 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.62e-14 | 628 | 871 | 74 | 291 | Crystal structure of the mutant S202W/I203F of the esterase E40 [uncultured bacterium],5GMS_B Crystal structure of the mutant S202W/I203F of the esterase E40 [uncultured bacterium] |
|
| 2.68e-14 | 628 | 871 | 76 | 293 | Crystal structure of the mutant M3+S202W/I203F of the esterase E40 [uncultured bacterium],5GMR_B Crystal structure of the mutant M3+S202W/I203F of the esterase E40 [uncultured bacterium],5GMR_C Crystal structure of the mutant M3+S202W/I203F of the esterase E40 [uncultured bacterium],5GMR_D Crystal structure of the mutant M3+S202W/I203F of the esterase E40 [uncultured bacterium] |
|
| 4.49e-14 | 637 | 833 | 66 | 242 | Crystal structure of Esterase/Lipase from uncultured bacterium [uncultured bacterium] |
|
| 5.23e-14 | 637 | 833 | 79 | 255 | Structural and Functional Analysis of a Hormone-Sensitive Lipase like EstE5 from a Metagenome Library [uncultured bacterium],3G9T_A Crystal structure of EstE5, was soaked by p-nitrophenyl butyrate for 5sec [uncultured bacterium],3G9U_A Crystal structure of EstE5, was soaked by p-nitrophenyl butyrate for 5min [uncultured bacterium],3G9Z_A Crystal structure of EstE5, was soaked by p-nitrophenyl caprylate [uncultured bacterium],3H17_A Crystal structure of EstE5-PMSF (I) [uncultured bacterium],3H18_A Crystal structure of EstE5-PMSF (II) [uncultured bacterium],3H19_A Crystal structure of EstE5, was soaked by methyl alcohol [uncultured bacterium],3H1A_A Crystal structure of EstE5, was soaked by ethyl alcohol [uncultured bacterium],3H1B_A Crystal structure of EstE5, was soaked by isopropyl alcohol [uncultured bacterium],3L1H_A Crystal structure of EstE5, was soaked by FeCl3 [uncultured bacterium],3L1I_A Crystal structure of EstE5, was soaked by CuSO4 [uncultured bacterium],3L1J_A Crystal structure of EstE5, was soaked by ZnSO4 [uncultured bacterium] |
|
| 8.89e-14 | 628 | 871 | 78 | 295 | Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_B Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_C Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_D Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_E Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_F Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_G Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples],4XVC_H Crystal structure of an esterase from the bacterial hormone-sensitive lipase (HSL) family [environmental samples] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.65e-09 | 614 | 794 | 87 | 255 | Arylacetamide deacetylase OS=Bos taurus OX=9913 GN=AADAC PE=2 SV=1 |
|
| 1.23e-08 | 640 | 794 | 83 | 224 | Carboxylesterase NlhH OS=Mycobacterium tuberculosis (strain CDC 1551 / Oshkosh) OX=83331 GN=nlhH PE=3 SV=1 |
|
| 1.23e-08 | 640 | 794 | 83 | 224 | Carboxylesterase NlhH OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=nlhH PE=1 SV=1 |
|
| 3.46e-08 | 635 | 833 | 69 | 248 | Esterase OS=Acinetobacter venetianus (strain ATCC 31012 / DSM 23050 / BCRC 14357 / CCUG 45561 / CIP 110063 / KCTC 2702 / LMG 19082 / RAG-1) OX=1191460 GN=est PE=3 SV=2 |
|
| 1.28e-06 | 641 | 794 | 107 | 255 | Arylacetamide deacetylase OS=Homo sapiens OX=9606 GN=AADAC PE=1 SV=5 |
SignalP and Lipop Annotations help
This protein is predicted as SP
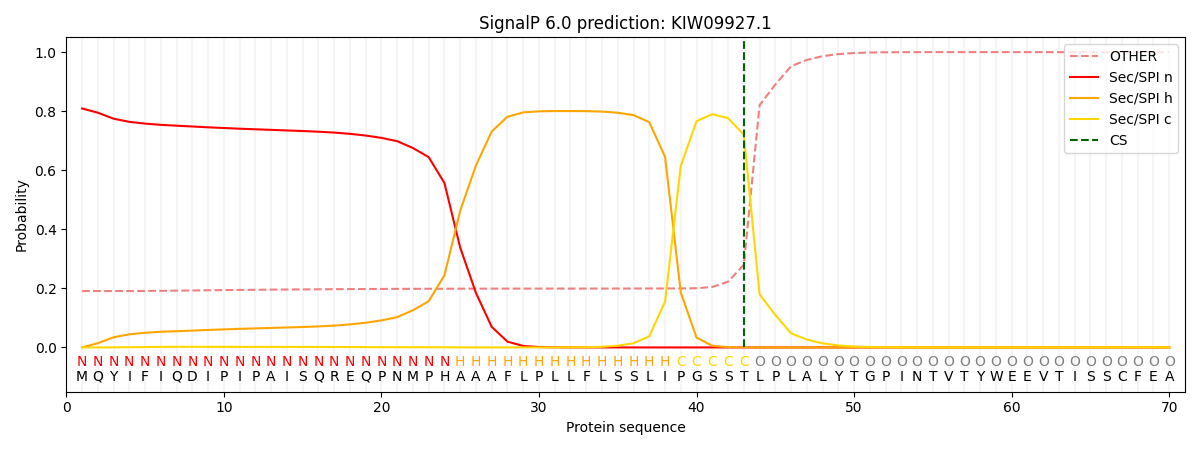
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.200551 | 0.799438 | CS pos: 43-44. Pr: 0.7202 |
