You are browsing environment: FUNGIDB
CAZyme Information: KFA55717.1
You are here: Home > Sequence: KFA55717.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Stachybotrys chartarum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Stachybotryaceae; Stachybotrys; Stachybotrys chartarum | |||||||||||
| CAZyme ID | KFA55717.1 | |||||||||||
| CAZy Family | GT21 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.26:1 | 3.2.1.80:2 | 3.2.1.26:1 | 3.2.1.80:2 | 3.2.1.26:1 | 3.2.1.7:10 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH32 | 33 | 295 | 2.5e-72 | 0.764505119453925 |
| GH32 | 926 | 1243 | 1e-71 | 0.9829351535836177 |
| CBM38 | 334 | 459 | 9.8e-34 | 0.9612403100775194 |
| CBM38 | 499 | 614 | 4.3e-32 | 0.9147286821705426 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 350134 | GH32_Inu-like | 5.96e-111 | 38 | 298 | 1 | 233 | glycoside hydrolase family 32 protein such as Aspergillus ficuum endo-inulinase (Inu2). This subfamily of glycosyl hydrolase family GH32 includes endo-inulinase (inu2, EC 3.2.1.7), exo-inulinase (Inu1, EC 3.2.1.80), invertase (EC 3.2.1.26), and levan fructotransferase (LftA, EC 4.2.2.16), among others. These enzymes cleave sucrose into fructose and glucose via beta-fructofuranosidase activity, producing invert sugar that is a mixture of dextrorotatory D-glucose and levorotatory D-fructose, thus named invertase (EC 3.2.1.26). These retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. These enzymes are predicted to display a 5-fold beta-propeller fold as found for GH43 and CH68. The breakdown of sucrose is widely used as a carbon or energy source by bacteria, fungi, and plants. Invertase is used commercially in the confectionery industry, since fructose has a sweeter taste than sucrose and a lower tendency to crystallize. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| 350134 | GH32_Inu-like | 1.21e-105 | 931 | 1236 | 1 | 289 | glycoside hydrolase family 32 protein such as Aspergillus ficuum endo-inulinase (Inu2). This subfamily of glycosyl hydrolase family GH32 includes endo-inulinase (inu2, EC 3.2.1.7), exo-inulinase (Inu1, EC 3.2.1.80), invertase (EC 3.2.1.26), and levan fructotransferase (LftA, EC 4.2.2.16), among others. These enzymes cleave sucrose into fructose and glucose via beta-fructofuranosidase activity, producing invert sugar that is a mixture of dextrorotatory D-glucose and levorotatory D-fructose, thus named invertase (EC 3.2.1.26). These retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. These enzymes are predicted to display a 5-fold beta-propeller fold as found for GH43 and CH68. The breakdown of sucrose is widely used as a carbon or energy source by bacteria, fungi, and plants. Invertase is used commercially in the confectionery industry, since fructose has a sweeter taste than sucrose and a lower tendency to crystallize. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| 214757 | Glyco_32 | 2.73e-103 | 926 | 1370 | 1 | 437 | Glycosyl hydrolases family 32. |
| 224536 | SacC | 1.31e-83 | 916 | 1396 | 23 | 475 | Sucrose-6-phosphate hydrolase SacC, GH32 family [Carbohydrate transport and metabolism]. |
| 395193 | Glyco_hydro_32N | 2.31e-81 | 926 | 1243 | 1 | 304 | Glycosyl hydrolases family 32 N-terminal domain. This domain corresponds to the N-terminal domain of glycosyl hydrolase family 32 which forms a five bladed beta propeller structure. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 592 | 1406 | 2 | 833 | |
| 6.95e-277 | 9 | 871 | 8 | 702 | |
| 2.28e-268 | 8 | 862 | 10 | 692 | |
| 7.19e-250 | 920 | 1403 | 19 | 502 | |
| 4.07e-244 | 24 | 871 | 28 | 702 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.79e-224 | 913 | 1408 | 20 | 515 | First crystal structure of an endo-inulinase, from Aspergillus ficuum: structural analysis and comparison with other GH32 enzymes. [Aspergillus ficuum],3SC7_X First crystal structure of an endo-inulinase, from Aspergillus ficuum: structural analysis and comparison with other GH32 enzymes. [Aspergillus ficuum] |
|
| 1.67e-164 | 24 | 863 | 3 | 509 | Crystal structure of exo-inulinase from Aspergillus awamori in spacegroup P21 [Aspergillus awamori],1Y9G_A Crystal structure of exo-inulinase from Aspergillus awamori complexed with fructose [Aspergillus awamori],1Y9M_A Crystal structure of exo-inulinase from Aspergillus awamori in spacegroup P212121 [Aspergillus awamori] |
|
| 3.33e-54 | 29 | 297 | 8 | 255 | Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_B Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_C Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_D Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_E Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_F Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_G Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C],4EQV_H Structure of Saccharomyces cerevisiae invertase [Saccharomyces cerevisiae S288C] |
|
| 3.91e-49 | 922 | 1248 | 10 | 323 | Chain A, Invertase [Schwanniomyces occidentalis],3KF3_B Chain B, Invertase [Schwanniomyces occidentalis] |
|
| 4.15e-49 | 922 | 1248 | 13 | 326 | Chain A, Invertase [Schwanniomyces occidentalis],3KF5_B Chain B, Invertase [Schwanniomyces occidentalis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.23e-245 | 24 | 871 | 28 | 702 | Putative glycosyl hydrolase ecdE OS=Aspergillus rugulosus OX=41736 GN=ecdE PE=3 SV=1 |
|
| 1.43e-227 | 920 | 1404 | 17 | 501 | Putative glycosyl hydrolase ecdF OS=Aspergillus rugulosus OX=41736 GN=ecdF PE=3 SV=1 |
|
| 2.84e-223 | 913 | 1408 | 20 | 515 | Extracellular endo-inulinase inuA OS=Aspergillus niger OX=5061 GN=inuA PE=1 SV=1 |
|
| 4.01e-223 | 913 | 1408 | 20 | 515 | Extracellular endo-inulinase inu2 OS=Aspergillus ficuum OX=5058 GN=inu2 PE=1 SV=1 |
|
| 1.24e-221 | 913 | 1408 | 20 | 515 | Extracellular endo-inulinase inuA OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=inuA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
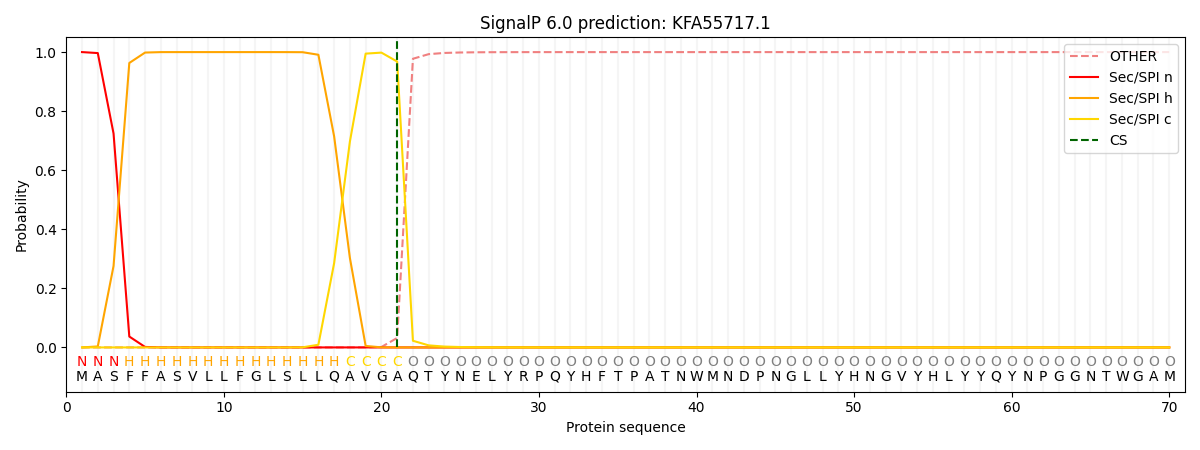
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000181 | 0.999821 | CS pos: 21-22. Pr: 0.9680 |
