You are browsing environment: FUNGIDB
CAZyme Information: KFA52727.1
You are here: Home > Sequence: KFA52727.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
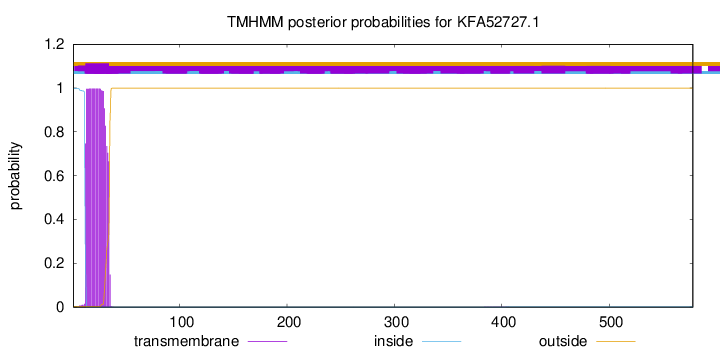
TMHMM annotations
Basic Information help
| Species | Stachybotrys chartarum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Stachybotryaceae; Stachybotrys; Stachybotrys chartarum | |||||||||||
| CAZyme ID | KFA52727.1 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.113:7 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH47 | 110 | 571 | 3.4e-159 | 0.9955156950672646 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396217 | Glyco_hydro_47 | 0.0 | 110 | 571 | 2 | 453 | Glycosyl hydrolase family 47. Members of this family are alpha-mannosidases that catalyze the hydrolysis of the terminal 1,2-linked alpha-D-mannose residues in the oligo-mannose oligosaccharide Man(9)(GlcNAc)(2). |
| 240427 | PTZ00470 | 4.22e-124 | 60 | 572 | 35 | 519 | glycoside hydrolase family 47 protein; Provisional |
| 224250 | YyaL | 1.75e-04 | 136 | 278 | 407 | 562 | Uncharacterized conserved protein YyaL, SSP411 family, contains thoiredoxin and six-hairpin glycosidase-like domains [General function prediction only]. |
| 271200 | LanM-like | 1.86e-04 | 191 | 313 | 598 | 705 | Cyclases involved in the biosynthesis of class II lantibiotics, and similar proteins. LanM-like proteins. LanM is a bifunctional enzyme, involved in the synthesis of class II lantibiotics. It is responsible for both the dehydration and the cyclization of the precursor-peptide during lantibiotic synthesis. The C-terminal domain shows similarity to LanC, the cyclase component of the lan operon, but the N terminus seems to be unrelated to the dehydratase, LanB. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 6.33e-232 | 7 | 572 | 9 | 577 | |
| 6.77e-231 | 7 | 572 | 8 | 566 | |
| 6.77e-231 | 7 | 572 | 8 | 566 | |
| 9.38e-227 | 44 | 573 | 39 | 570 | |
| 3.68e-225 | 44 | 576 | 37 | 571 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.30e-71 | 100 | 569 | 3 | 471 | Penicillium citrinum alpha-1,2-mannosidase complex with glycerol [Penicillium citrinum],2RI8_B Penicillium citrinum alpha-1,2-mannosidase complex with glycerol [Penicillium citrinum],2RI9_A Penicillium citrinum alpha-1,2-mannosidase in complex with a substrate analog [Penicillium citrinum],2RI9_B Penicillium citrinum alpha-1,2-mannosidase in complex with a substrate analog [Penicillium citrinum] |
|
| 1.97e-71 | 113 | 569 | 16 | 449 | Crystal structure of the class I human endoplasmic reticulum 1,2-alpha-mannosidase and Man9GlcNAc2-PA complex [Homo sapiens] |
|
| 2.30e-71 | 113 | 569 | 21 | 454 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase [Homo sapiens] |
|
| 2.43e-71 | 113 | 569 | 21 | 454 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With 1-Deoxymannojirimycin [Homo sapiens],1FO3_A Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With Kifunensine [Homo sapiens] |
|
| 3.18e-71 | 100 | 569 | 38 | 506 | Structure of P. citrinum alpha 1,2-mannosidase reveals the basis for differences in specificity of the ER and Golgi Class I enzymes [Penicillium citrinum],1KKT_B Structure of P. citrinum alpha 1,2-mannosidase reveals the basis for differences in specificity of the ER and Golgi Class I enzymes [Penicillium citrinum],1KRE_A Structure Of P. Citrinum Alpha 1,2-Mannosidase Reveals The Basis For Differences In Specificity Of The Er And Golgi Class I Enzymes [Penicillium citrinum],1KRE_B Structure Of P. Citrinum Alpha 1,2-Mannosidase Reveals The Basis For Differences In Specificity Of The Er And Golgi Class I Enzymes [Penicillium citrinum],1KRF_A Structure Of P. Citrinum Alpha 1,2-mannosidase Reveals The Basis For Differences In Specificity Of The Er And Golgi Class I Enzymes [Penicillium citrinum],1KRF_B Structure Of P. Citrinum Alpha 1,2-mannosidase Reveals The Basis For Differences In Specificity Of The Er And Golgi Class I Enzymes [Penicillium citrinum] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7.71e-82 | 92 | 570 | 35 | 513 | Mannosyl-oligosaccharide alpha-1,2-mannosidase OS=Coccidioides posadasii (strain RMSCC 757 / Silveira) OX=443226 GN=CPSG_02648 PE=1 SV=1 |
|
| 1.14e-76 | 85 | 570 | 24 | 506 | Probable mannosyl-oligosaccharide alpha-1,2-mannosidase ARB_00035 OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_00035 PE=1 SV=1 |
|
| 1.69e-76 | 95 | 570 | 28 | 507 | Probable mannosyl-oligosaccharide alpha-1,2-mannosidase 1B OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=mns1B PE=3 SV=1 |
|
| 2.09e-73 | 97 | 570 | 37 | 509 | Probable mannosyl-oligosaccharide alpha-1,2-mannosidase 1B OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=mns1B PE=3 SV=1 |
|
| 2.58e-73 | 95 | 570 | 32 | 506 | Probable mannosyl-oligosaccharide alpha-1,2-mannosidase 1B OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=mns1B PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000045 | 0.000003 |