You are browsing environment: FUNGIDB
CAZyme Information: KFA51353.1
You are here: Home > Sequence: KFA51353.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Stachybotrys chartarum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Stachybotryaceae; Stachybotrys; Stachybotrys chartarum | |||||||||||
| CAZyme ID | KFA51353.1 | |||||||||||
| CAZy Family | GH16 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 48 | 265 | 1e-54 | 0.4672489082969432 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396238 | FAD_binding_4 | 1.07e-17 | 58 | 194 | 1 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 223354 | GlcD | 2.24e-17 | 52 | 466 | 26 | 448 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 223882 | MurB | 1.85e-07 | 49 | 253 | 11 | 194 | UDP-N-acetylenolpyruvoylglucosamine reductase [Cell wall/membrane/envelope biogenesis]. |
| 369658 | BBE | 6.22e-06 | 433 | 468 | 1 | 38 | Berberine and berberine like. This domain is found in the berberine bridge and berberine bridge- like enzymes which are involved in the biosynthesis of numerous isoquinoline alkaloids. They catalyze the transformation of the N-methyl group of (S)-reticuline into the C-8 berberine bridge carbon of (S)-scoulerine. |
| 273751 | FAD_lactone_ox | 2.82e-04 | 102 | 232 | 57 | 186 | sugar 1,4-lactone oxidases. This model represents a family of at least two different sugar 1,4 lactone oxidases, both involved in synthesizing ascorbic acid or a derivative. These include L-gulonolactone oxidase (EC 1.1.3.8) from rat and D-arabinono-1,4-lactone oxidase (EC 1.1.3.37) from Saccharomyces cerevisiae. Members are proposed to have the cofactor FAD covalently bound at a site specified by Prosite motif PS00862; OX2_COVAL_FAD; 1. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.67e-23 | 55 | 469 | 60 | 485 | |
| 5.10e-22 | 55 | 475 | 17 | 442 | |
| 5.38e-22 | 58 | 468 | 70 | 489 | |
| 1.79e-18 | 55 | 468 | 45 | 463 | |
| 6.46e-18 | 10 | 468 | 12 | 489 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.32e-24 | 34 | 468 | 4 | 453 | The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_B The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_C The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_D The crystal structure of EncM T139V mutant [Streptomyces maritimus] |
|
| 4.48e-24 | 34 | 468 | 4 | 453 | The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_B The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_C The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_D The crystal structure of EncM V135T mutant [Streptomyces maritimus] |
|
| 5.81e-24 | 50 | 475 | 31 | 455 | Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 3 (P1) [Paenarthrobacter nicotinovorans],2BVF_B Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 3 (P1) [Paenarthrobacter nicotinovorans],2BVG_A Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 1 (P21) [Paenarthrobacter nicotinovorans],2BVG_B Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 1 (P21) [Paenarthrobacter nicotinovorans],2BVG_C Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 1 (P21) [Paenarthrobacter nicotinovorans],2BVG_D Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 1 (P21) [Paenarthrobacter nicotinovorans],2BVH_A Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 2 (P21) [Paenarthrobacter nicotinovorans],2BVH_B Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 2 (P21) [Paenarthrobacter nicotinovorans],2BVH_C Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 2 (P21) [Paenarthrobacter nicotinovorans],2BVH_D Crystal structure of 6-hydoxy-D-nicotine oxidase from Arthrobacter nicotinovorans. Crystal Form 2 (P21) [Paenarthrobacter nicotinovorans] |
|
| 6.05e-24 | 34 | 468 | 4 | 453 | The crystal structure of EncM H138T mutant [Streptomyces maritimus],6FYE_B The crystal structure of EncM H138T mutant [Streptomyces maritimus] |
|
| 8.16e-24 | 34 | 468 | 4 | 453 | The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_B The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_C The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_D The crystal structure of EncM V135M mutant [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.52e-65 | 39 | 468 | 52 | 496 | FAD-linked oxidoreductase OXR1 OS=Magnaporthe oryzae (strain 70-15 / ATCC MYA-4617 / FGSC 8958) OX=242507 GN=OXR1 PE=1 SV=1 |
|
| 1.35e-56 | 24 | 468 | 33 | 489 | FAD-linked oxidoreductase srdI OS=Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) OX=367110 GN=srdI PE=2 SV=1 |
|
| 1.70e-55 | 6 | 473 | 16 | 505 | FAD-linked oxidoreductase virI OS=Hypocrea virens (strain Gv29-8 / FGSC 10586) OX=413071 GN=virI PE=3 SV=1 |
|
| 2.13e-54 | 49 | 459 | 52 | 471 | FAD-linked oxidoreductase azaL OS=Aspergillus niger (strain ATCC 1015 / CBS 113.46 / FGSC A1144 / LSHB Ac4 / NCTC 3858a / NRRL 328 / USDA 3528.7) OX=380704 GN=azaL PE=2 SV=2 |
|
| 5.52e-52 | 45 | 474 | 25 | 449 | FAD-linked oxidoreductase pyvE OS=Aspergillus violaceofuscus (strain CBS 115571) OX=1450538 GN=pyvE PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
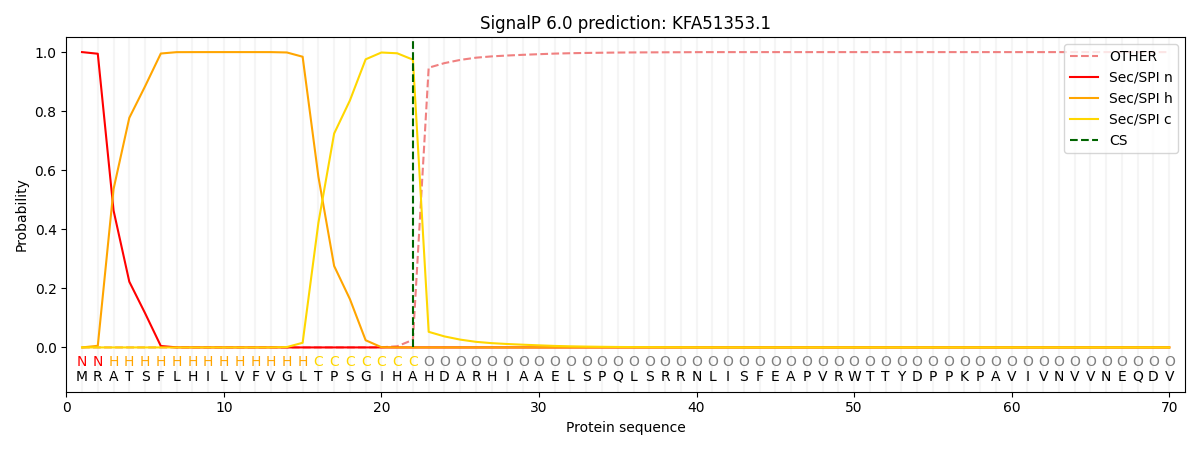
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000277 | 0.999710 | CS pos: 22-23. Pr: 0.9744 |
