You are browsing environment: FUNGIDB
CAZyme Information: KDQ28851.1
You are here: Home > Sequence: KDQ28851.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Pleurotus ostreatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Agaricomycetes; ; Pleurotaceae; Pleurotus; Pleurotus ostreatus | |||||||||||
| CAZyme ID | KDQ28851.1 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA3 | 75 | 406 | 4.9e-55 | 0.6954225352112676 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 153335 | F-BAR_PombeCdc15_like | 2.14e-117 | 459 | 693 | 1 | 236 | The F-BAR (FES-CIP4 Homology and Bin/Amphiphysin/Rvs) domain of Schizosaccharomyces pombe Cdc15, and similar proteins. F-BAR domains are dimerization modules that bind and bend membranes and are found in proteins involved in membrane dynamics and actin reorganization. This subfamily is composed of Schizosaccharomyces pombe Cdc15 and Imp2, and similar proteins. These proteins contain an N-terminal F-BAR domain and a C-terminal SH3 domain. S. pombe Cdc15 and Imp2 play both distinct and overlapping roles in the maintenance and strengthening of the contractile ring at the division site, which is required in cell division. Cdc15 is a component of the actomyosin ring and is required in normal cytokinesis. Imp2 colocalizes with the medial ring during septation and is required for normal septation. F-BAR domains form banana-shaped dimers with a positively-charged concave surface that binds to negatively-charged lipid membranes. They can induce membrane deformation in the form of long tubules. |
| 153331 | F-BAR_PSTPIP | 3.32e-45 | 464 | 692 | 6 | 238 | The F-BAR (FES-CIP4 Homology and Bin/Amphiphysin/Rvs) domain of Proline-Serine-Threonine Phosphatase-Interacting Proteins. F-BAR domains are dimerization modules that bind and bend membranes and are found in proteins involved in membrane dynamics and actin reorganization. Vetebrates contain two Proline-Serine-Threonine Phosphatase-Interacting Proteins (PSTPIPs), PSTPIP1 and PSTPIP2. PSTPIPs are mainly expressed in hematopoietic cells and are involved in the regulation of cell adhesion and motility. Mutations in PSTPIPs have been shown to cause autoinflammatory disorders. PSTPIP1 contains an N-terminal F-BAR domain, PEST motifs, and a C-terminal SH3 domain, while PSTPIP2 contains only the N-terminal F-BAR domain. F-BAR domains form banana-shaped dimers with a positively-charged concave surface that binds to negatively-charged lipid membranes. They can induce membrane deformation in the form of long tubules. |
| 235000 | PRK02106 | 9.94e-39 | 148 | 401 | 204 | 466 | choline dehydrogenase; Validated |
| 225186 | BetA | 5.87e-33 | 12 | 414 | 40 | 476 | Choline dehydrogenase or related flavoprotein [Lipid transport and metabolism, General function prediction only]. |
| 153355 | F-BAR_PSTPIP1 | 6.75e-31 | 464 | 696 | 6 | 242 | The F-BAR (FES-CIP4 Homology and Bin/Amphiphysin/Rvs) domain of Proline-Serine-Threonine Phosphatase-Interacting Protein 1. F-BAR domains are dimerization modules that bind and bend membranes and are found in proteins involved in membrane dynamics and actin reorganization. Proline-Serine-Threonine Phosphatase-Interacting Protein 1 (PSTPIP1), also known as CD2 Binding Protein 1 (CD2BP1), is mainly expressed in hematopoietic cells. It is a binding partner of the cell surface receptor CD2 and PTP-PEST, a tyrosine phosphatase which functions in cell motility and Rac1 regulation. It also plays a role in the activation of the Wiskott-Aldrich syndrome protein (WASP), which couples actin rearrangement and T cell activation. Mutations in the gene encoding PSTPIP1 cause the autoinflammatory disorder known as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome. PSTPIP1 contains an N-terminal F-BAR domain, PEST motifs, and a C-terminal SH3 domain. F-BAR domains form banana-shaped dimers with a positively-charged concave surface that binds to negatively-charged lipid membranes. They can induce membrane deformation in the form of long tubules. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.67e-52 | 145 | 397 | 23 | 331 | |
| 5.93e-49 | 3 | 409 | 6 | 518 | |
| 7.40e-41 | 149 | 409 | 18 | 323 | |
| 8.25e-37 | 138 | 406 | 13 | 331 | |
| 1.46e-31 | 18 | 400 | 73 | 548 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.25e-50 | 444 | 726 | 1 | 282 | Structure of the Imp2 F-BAR domain [Schizosaccharomyces pombe 972h-],5C1F_B Structure of the Imp2 F-BAR domain [Schizosaccharomyces pombe 972h-] |
|
| 1.59e-48 | 450 | 726 | 1 | 278 | Chain A, Cell division control protein 15 [Schizosaccharomyces pombe 972h-],6XJ1_B Chain B, Cell division control protein 15 [Schizosaccharomyces pombe 972h-] |
|
| 3.38e-30 | 444 | 726 | 1 | 290 | Chain A, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens],7AAL_B Chain B, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens] |
|
| 4.58e-30 | 444 | 726 | 1 | 290 | Chain A, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens],7AAM_B Chain B, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens],7AAN_A Chain A, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens],7AAN_B Chain B, Proline-serine-threonine phosphatase-interacting protein 1 [Homo sapiens] |
|
| 2.45e-20 | 76 | 421 | 112 | 518 | Crystal structure of Aspergillus flavus FAD glucose dehydrogenase [Aspergillus flavus NRRL3357],4YNU_A Crystal structure of Aspergillus flavus FADGDH in complex with D-glucono-1,5-lactone [Aspergillus flavus NRRL3357] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.54e-45 | 436 | 726 | 1 | 292 | Septation protein imp2 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=imp2 PE=1 SV=1 |
|
| 2.93e-43 | 448 | 726 | 17 | 296 | Cell division control protein 15 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=cdc15 PE=1 SV=2 |
|
| 1.92e-28 | 453 | 726 | 7 | 287 | Proline-serine-threonine phosphatase-interacting protein 1 OS=Mus musculus OX=10090 GN=Pstpip1 PE=1 SV=1 |
|
| 3.80e-27 | 453 | 726 | 7 | 287 | Proline-serine-threonine phosphatase-interacting protein 1 OS=Homo sapiens OX=9606 GN=PSTPIP1 PE=1 SV=1 |
|
| 3.03e-24 | 464 | 739 | 20 | 303 | Proline-serine-threonine phosphatase-interacting protein 2 OS=Mus musculus OX=10090 GN=Pstpip2 PE=1 SV=4 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
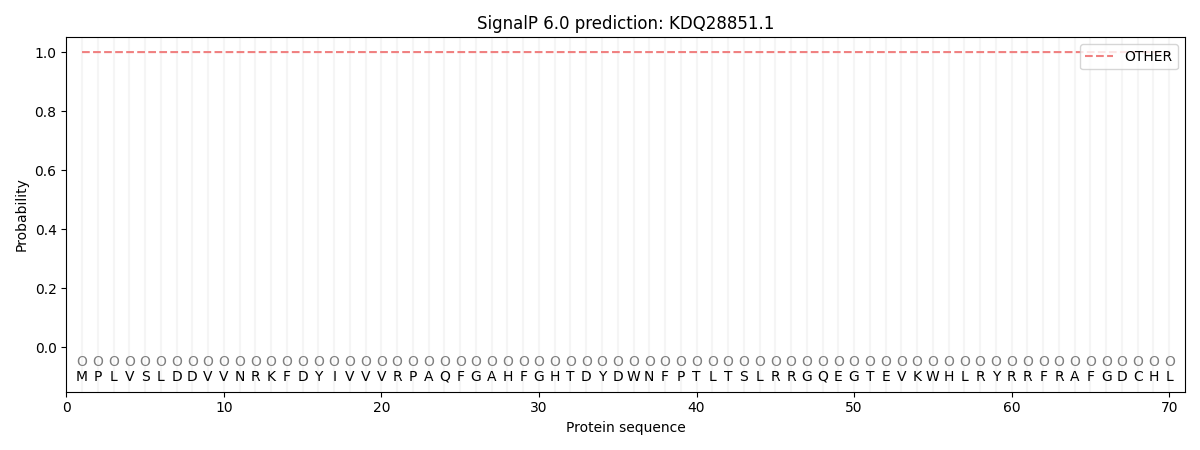
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000068 | 0.000000 |
