You are browsing environment: FUNGIDB
CAZyme Information: KAG2006145.1
You are here: Home > Sequence: KAG2006145.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Coprinopsis cinerea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Agaricomycetes; ; Psathyrellaceae; Coprinopsis; Coprinopsis cinerea | |||||||||||
| CAZyme ID | KAG2006145.1 | |||||||||||
| CAZy Family | AA9 | |||||||||||
| CAZyme Description | Peroxidase Precursor | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 1.11.1.16:9 | 1.11.1.13:5 | 1.11.1.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 45 | 304 | 1.9e-88 | 0.9921568627450981 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173826 | ligninase | 6.37e-172 | 29 | 357 | 1 | 328 | Ligninase and other manganese-dependent fungal peroxidases. Ligninases and related extracellular fungal peroxidases belong to class II of the plant heme-dependent peroxidase superfamily. All members of the superfamily share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Class II peroxidases are fungal glycoproteins that have been implicated in the oxidative breakdown of lignin, the main cell wall component of woody plants. They contain four conserved disulphide bridges and two conserved calcium binding sites. |
| 173823 | plant_peroxidase_like | 1.58e-39 | 47 | 300 | 2 | 254 | Heme-dependent peroxidases similar to plant peroxidases. Along with animal peroxidases, these enzymes belong to a group of peroxidases containing a heme prosthetic group (ferriprotoporphyrin IX), which catalyzes a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. The plant peroxidase-like superfamily is found in all three kingdoms of life and carries out a variety of biosynthetic and degradative functions. Several sub-families can be identified. Class I includes intracellular peroxidases present in fungi, plants, archaea and bacteria, called catalase-peroxidases, that can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. Catalase-peroxidases are typically comprised of two homologous domains that probably arose via a single gene duplication event. Class II includes ligninase and other extracellular fungal peroxidases, while class III is comprised of classic extracellular plant peroxidases, like horseradish peroxidase. |
| 403189 | Peroxidase_ext | 3.35e-27 | 288 | 358 | 1 | 72 | Fungal peroxidase extension region. This region is found as an extension to a haem peroxidase domain in some fungi. This region is about 80 amino acids in length and forms an extended structure on the surface of the peroxidase domain pfam00141. |
| 395089 | peroxidase | 1.21e-14 | 68 | 284 | 18 | 187 | Peroxidase. |
| 173827 | secretory_peroxidase | 3.17e-07 | 52 | 303 | 2 | 282 | Horseradish peroxidase and related secretory plant peroxidases. Secretory peroxidases belong to class III of the plant heme-dependent peroxidase superfamily. All members of the superfamily share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Class III peroxidases are found in the extracellular space or in the vacuole in plants where they have been implicated in hydrogen peroxide detoxification, auxin catabolism and lignin biosynthesis, and stress response. Class III peroxidases contain four conserved disulphide bridges and two conserved calcium binding sites. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.16e-261 | 1 | 363 | 1 | 363 | |
| 1.65e-261 | 1 | 363 | 1 | 363 | |
| 3.31e-259 | 1 | 363 | 1 | 364 | |
| 5.39e-143 | 30 | 359 | 28 | 355 | |
| 1.38e-129 | 9 | 359 | 7 | 357 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.89e-250 | 21 | 363 | 1 | 343 | Chain A, Peroxidase [Coprinopsis cinerea],1LYK_B Chain B, Peroxidase [Coprinopsis cinerea] |
|
| 2.41e-248 | 21 | 363 | 1 | 344 | Crystal structure of the fungal peroxidase from Arthromyces ramosus at 1.9 angstroms resolution: structural comparisons with the lignin and cytochrome C peroxidases [Penicillium janthinellum],1ARU_A Crystal Structures Of Cyanide-And Triiodide-Bound Forms Of Arthromyces Ramosus Peroxidase At Different Ph Values. Perturbations Of Active Site Residues And Their Implication In Enzyme Catalysis [Agaricales sp. 'Arthromyces ramosus'],1ARV_A Crystal Structures Of Cyanide-And Triiodide-Bound Forms Of Arthromyces Ramosus Peroxidase At Different Ph Values. Perturbations Of Active Site Residues And Their Implication In Enzyme Catalysis [Agaricales sp. 'Arthromyces ramosus'],1ARW_A Crystal Structures Of Cyanide-And Triiodide-Bound Forms Of Arthromyces Ramosus Peroxidase At Different Ph Values. Perturbations Of Active Site Residues And Their Implication In Enzyme Catalysis [Agaricales sp. 'Arthromyces ramosus'],1ARX_A Crystal Structures Of Cyanide-And Triiodide-Bound Forms Of Arthromyces Ramosus Peroxidase At Different Ph Values. Perturbations Of Active Site Residues And Their Implication In Enzyme Catalysis [Agaricales sp. 'Arthromyces ramosus'],1ARY_A Crystal Structures Of Cyanide-And Triiodide-Bound Forms Of Arthromyces Ramosus Peroxidase At Different Ph Values. Perturbations Of Active Site Residues And Their Implication In Enzyme Catalysis [Agaricales sp. 'Arthromyces ramosus'],1C8I_A BINDING MODE OF HYDROXYLAMINE TO ARTHROMYCES RAMOSUS PEROXIDASE [Agaricales sp. 'Arthromyces ramosus'],1CK6_A BINDING MODE OF SALICYLHYDROXAMIC ACID TO ARTHROMYCES RAMOSUS PEROXIDASE [Agaricales sp. 'Arthromyces ramosus'],1GZA_A PEROXIDASE [Agaricales sp. 'Arthromyces ramosus'],1GZB_A PEROXIDASE [Agaricales sp. 'Arthromyces ramosus'],1HSR_A BINDING MODE OF BENZHYDROXAMIC ACID TO ARTHROMYCES RAMOSUS PEROXIDASE [Agaricales sp. 'Arthromyces ramosus'],2E39_A Crystal structure of the CN-bound form of Arthromyces ramosus peroxidase at 1.3 Angstroms resolution [Agaricales sp. 'Arthromyces ramosus'],2E3A_A Crystal structure of the NO-bound form of Arthromyces ramosus peroxidase at 1.3 Angstroms resolution [Agaricales sp. 'Arthromyces ramosus'],2E3B_A Crystal structure of the HA-bound form of Arthromyces ramosus peroxidase at 1.3 Angstroms resolution [Agaricales sp. 'Arthromyces ramosus'] |
|
| 4.68e-248 | 21 | 363 | 1 | 343 | Chain A, Peroxidase [Coprinopsis cinerea],1LY9_B Chain B, Peroxidase [Coprinopsis cinerea],1LYC_A Chain A, Peroxidase [Coprinopsis cinerea],1LYC_B Chain B, Peroxidase [Coprinopsis cinerea] |
|
| 6.41e-248 | 22 | 363 | 1 | 342 | Chain A, PEROXIDASE [Coprinopsis cinerea],1H3J_B Chain B, PEROXIDASE [Coprinopsis cinerea] |
|
| 1.06e-244 | 21 | 363 | 1 | 343 | Chain A, Peroxidase [Coprinopsis cinerea],1LY8_B Chain B, Peroxidase [Coprinopsis cinerea] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.56e-263 | 1 | 363 | 1 | 363 | Peroxidase OS=Coprinopsis cinerea (strain Okayama-7 / 130 / ATCC MYA-4618 / FGSC 9003) OX=240176 GN=CIP1 PE=3 SV=1 |
|
| 2.92e-262 | 1 | 363 | 1 | 363 | Peroxidase OS=Coprinopsis cinerea OX=5346 GN=CIP1 PE=1 SV=2 |
|
| 5.87e-260 | 1 | 363 | 1 | 364 | Peroxidase OS=Arthromyces ramosus OX=5451 PE=1 SV=3 |
|
| 2.10e-105 | 30 | 363 | 32 | 359 | Versatile peroxidase VPL2 OS=Pleurotus eryngii OX=5323 GN=vpl2 PE=1 SV=1 |
|
| 7.95e-105 | 1 | 363 | 1 | 368 | Versatile peroxidase VPS1 OS=Pleurotus eryngii OX=5323 GN=vps1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
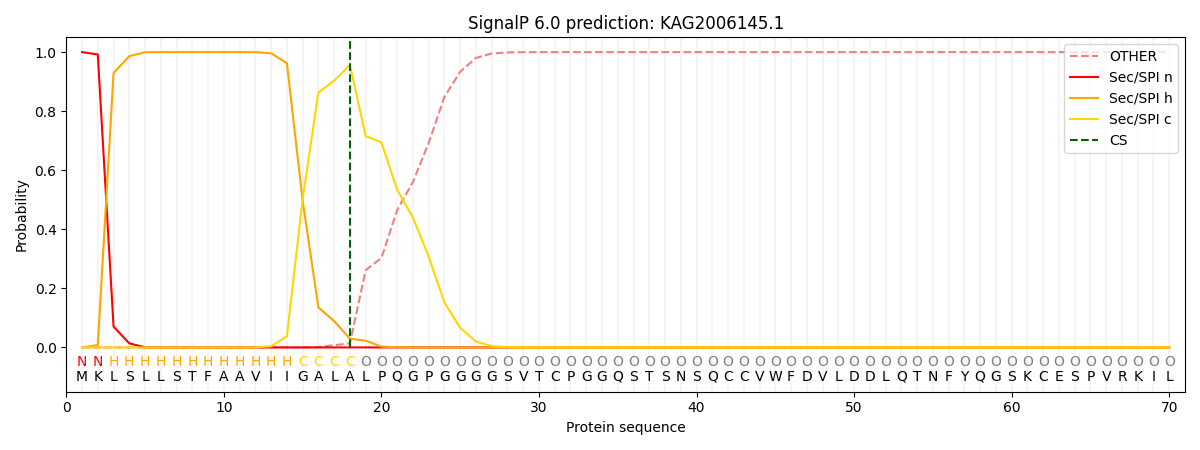
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000256 | 0.999698 | CS pos: 18-19. Pr: 0.9565 |
