You are browsing environment: FUNGIDB
CAZyme Information: KAB8206169.1
You are here: Home > Sequence: KAB8206169.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus parasiticus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus parasiticus | |||||||||||
| CAZyme ID | KAB8206169.1 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | GDSL lipase/esterase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE16 | 32 | 288 | 2.7e-111 | 0.9925093632958801 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 238882 | fatty_acyltransferase_like | 1.43e-59 | 31 | 288 | 1 | 267 | Fatty acyltransferase-like subfamily of the SGNH hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. Might catalyze fatty acid transfer between phosphatidylcholine and sterols. |
| 238875 | SGNH_plant_lipase_like | 2.42e-29 | 90 | 279 | 79 | 302 | SGNH_plant_lipase_like, a plant specific subfamily of the SGNH-family of hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| 395531 | Lipase_GDSL | 9.36e-13 | 89 | 286 | 46 | 222 | GDSL-like Lipase/Acylhydrolase. |
| 225780 | COG3240 | 2.33e-07 | 8 | 293 | 9 | 334 | Phospholipase/lecithinase/hemolysin [Lipid transport and metabolism, General function prediction only]. |
| 238883 | Triacylglycerol_lipase_like | 2.52e-06 | 32 | 283 | 4 | 272 | Triacylglycerol lipase-like subfamily of the SGNH hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. Members of this subfamily might hydrolyze triacylglycerol into diacylglycerol and fatty acid anions. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.50e-222 | 1 | 298 | 1 | 298 | |
| 1.50e-222 | 1 | 298 | 1 | 298 | |
| 1.50e-222 | 1 | 298 | 1 | 298 | |
| 9.81e-218 | 1 | 298 | 1 | 313 | |
| 5.59e-159 | 1 | 235 | 1 | 250 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.03e-08 | 35 | 288 | 15 | 281 | Crystal structure of ChoE D285N mutant in complex with acetate and thiocholine [Pseudomonas aeruginosa PAO1],6UQZ_B Crystal structure of ChoE D285N mutant in complex with acetate and thiocholine [Pseudomonas aeruginosa PAO1] |
|
| 3.36e-08 | 73 | 288 | 49 | 281 | Crystal structure of ChoE D285N mutant acyl-enzyme [Pseudomonas aeruginosa PAO1],6UR0_B Crystal structure of ChoE D285N mutant acyl-enzyme [Pseudomonas aeruginosa PAO1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.01e-68 | 18 | 286 | 21 | 291 | Acetylesterase OS=Myceliophthora thermophila (strain ATCC 42464 / BCRC 31852 / DSM 1799) OX=573729 GN=aes1 PE=3 SV=1 |
|
| 1.01e-68 | 18 | 286 | 21 | 291 | Acetylesterase OS=Thermothelomyces thermophilus OX=78579 GN=aes1 PE=1 SV=1 |
|
| 4.72e-11 | 16 | 266 | 25 | 311 | GDSL esterase/lipase LIP-4 OS=Arabidopsis thaliana OX=3702 GN=LIP4 PE=2 SV=1 |
|
| 1.01e-07 | 136 | 254 | 168 | 298 | GDSL esterase/lipase At4g01130 OS=Arabidopsis thaliana OX=3702 GN=At4g01130 PE=2 SV=1 |
|
| 2.71e-07 | 124 | 290 | 259 | 443 | Anther-specific proline-rich protein APG (Fragment) OS=Brassica napus OX=3708 GN=APG PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
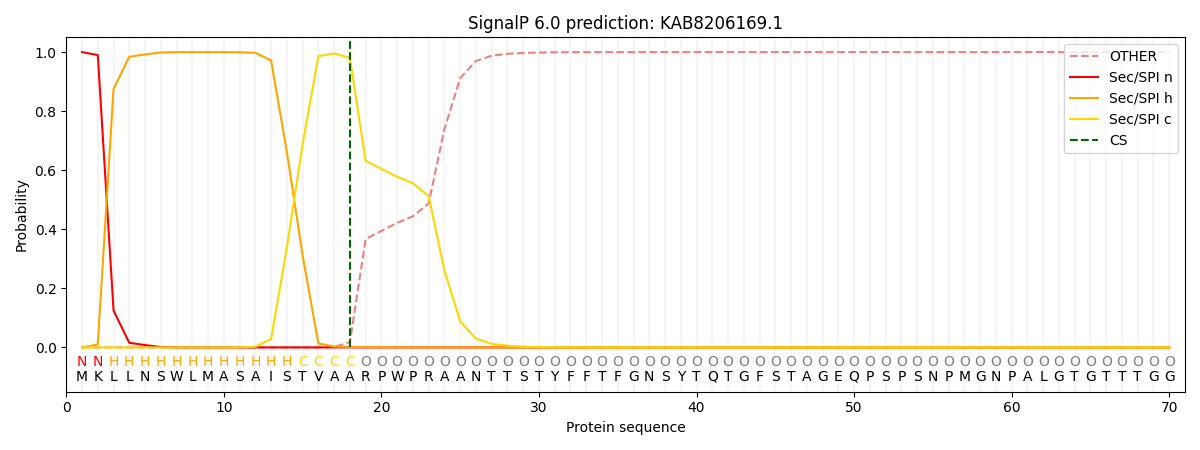
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000234 | 0.999725 | CS pos: 18-19. Pr: 0.9807 |
