You are browsing environment: FUNGIDB
CAZyme Information: I314_02998-t35_1-p1
You are here: Home > Sequence: I314_02998-t35_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Cryptococcus gattii VGIII | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Arthropoda; Insecta; ; Eriococcidae; Cryptococcus; Cryptococcus gattii VGIII | |||||||||||
| CAZyme ID | I314_02998-t35_1-p1 | |||||||||||
| CAZy Family | GH3 | |||||||||||
| CAZyme Description | chitinase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH18 | 127 | 485 | 1.4e-46 | 0.9358108108108109 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395573 | Glyco_hydro_18 | 1.36e-38 | 128 | 484 | 1 | 306 | Glycosyl hydrolases family 18. |
| 214753 | Glyco_18 | 1.18e-31 | 130 | 484 | 3 | 333 | Glyco_18 domain. |
| 119349 | GH18_chitinase-like | 4.24e-28 | 129 | 319 | 1 | 177 | The GH18 (glycosyl hydrolase, family 18) type II chitinases hydrolyze chitin, an abundant polymer of beta-1,4-linked N-acetylglucosamine (GlcNAc) which is a major component of the cell wall of fungi and the exoskeleton of arthropods. Chitinases have been identified in viruses, bacteria, fungi, protozoan parasites, insects, and plants. The structure of the GH18 domain is an eight-stranded beta/alpha barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. The GH18 family includes chitotriosidase, chitobiase, hevamine, zymocin-alpha, narbonin, SI-CLP (stabilin-1 interacting chitinase-like protein), IDGF (imaginal disc growth factor), CFLE (cortical fragment-lytic enzyme) spore hydrolase, the type III and type V plant chitinases, the endo-beta-N-acetylglucosaminidases, and the chitolectins. The GH85 (glycosyl hydrolase, family 85) ENGases (endo-beta-N-acetylglucosaminidases) are closely related to the GH18 chitinases and are included in this alignment model. |
| 119351 | GH18_chitolectin_chitotriosidase | 1.41e-26 | 157 | 382 | 26 | 260 | This conserved domain family includes a large number of catalytically inactive chitinase-like lectins (chitolectins) including YKL-39, YKL-40 (HCGP39), YM1, oviductin, and AMCase (acidic mammalian chitinase), as well as catalytically active chitotriosidases. The conserved domain is an eight-stranded alpha/beta barrel fold belonging to the family 18 glycosyl hydrolases. The fold has a pronounced active-site cleft at the C-terminal end of the beta-barrel. The chitolectins lack a key active site glutamate (the proton donor required for hydrolytic activity) but retain highly conserved residues involved in oligosaccharide binding. Chitotriosidase is a chitinolytic enzyme expressed in maturing macrophages, which suggests that it plays a part in antimicrobial defense. Chitotriosidase hydrolyzes chitotriose, as well as colloidal chitin to yield chitobiose and is therefore considered an exochitinase. Chitotriosidase occurs in two major forms, the large form being converted to the small form by either RNA or post-translational processing. Although the small form, containing the chitinase domain alone, is sufficient for the chitinolytic activity, the additional C-terminal chitin-binding domain of the large form plays a role in processing colloidal chitin. The chitotriosidase gene is nonessential in humans, as about 35% of the population are heterozygous and 6% homozygous for an inactivated form of the gene. HCGP39 is a 39-kDa human cartilage glycoprotein thought to play a role in connective tissue remodeling and defense against pathogens. |
| 119365 | GH18_chitinase | 8.19e-26 | 150 | 360 | 17 | 261 | The GH18 (glycosyl hydrolases, family 18) type II chitinases hydrolyze chitin, an abundant polymer of N-acetylglucosamine and have been identified in bacteria, fungi, insects, plants, viruses, and protozoan parasites. The structure of this domain is an eight-stranded alpha/beta barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.83e-314 | 1 | 501 | 1 | 501 | |
| 7.27e-256 | 51 | 484 | 75 | 498 | |
| 6.84e-233 | 126 | 484 | 161 | 509 | |
| 7.08e-233 | 119 | 484 | 155 | 510 | |
| 9.70e-233 | 126 | 484 | 161 | 509 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.02e-13 | 198 | 390 | 84 | 291 | Chain AAA, Peroxiredoxin [Clostridioides difficile 630] |
|
| 1.16e-13 | 198 | 390 | 103 | 310 | Chain AAA, Peroxiredoxin [Clostridioides difficile 630] |
|
| 4.75e-12 | 205 | 483 | 77 | 345 | Chain A, Probable endochitinase [Caenorhabditis elegans] |
|
| 4.80e-12 | 205 | 483 | 78 | 346 | Chain A, Probable endochitinase [Caenorhabditis elegans] |
|
| 6.77e-12 | 205 | 480 | 90 | 346 | Crystal structure of a native chitinase from Aspergillus fumigatus YJ-407 [Aspergillus fumigatus],1WNO_B Crystal structure of a native chitinase from Aspergillus fumigatus YJ-407 [Aspergillus fumigatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.60e-12 | 158 | 483 | 69 | 387 | Probable chitinase 2 OS=Drosophila melanogaster OX=7227 GN=Cht2 PE=1 SV=1 |
|
| 1.95e-11 | 158 | 483 | 50 | 363 | Acidic mammalian chitinase OS=Bos taurus OX=9913 GN=CHIA PE=1 SV=1 |
|
| 4.05e-11 | 205 | 480 | 128 | 384 | Endochitinase B1 OS=Neosartorya fumigata OX=746128 GN=chiB1 PE=1 SV=1 |
|
| 4.05e-11 | 205 | 480 | 128 | 384 | Endochitinase B1 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=chiB1 PE=3 SV=1 |
|
| 4.42e-11 | 205 | 483 | 129 | 397 | Probable endochitinase OS=Caenorhabditis elegans OX=6239 GN=cht-1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
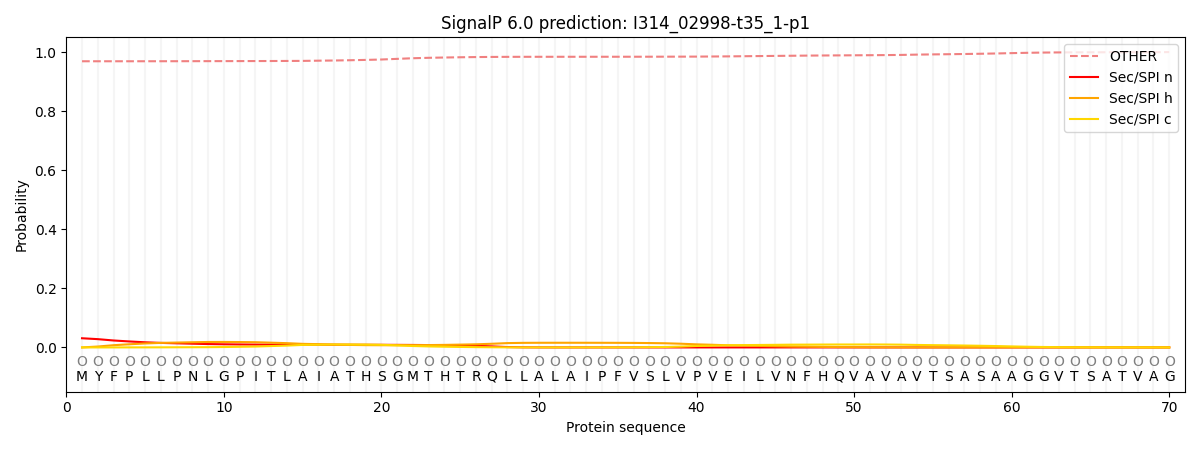
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.971589 | 0.028420 |
