You are browsing environment: FUNGIDB
CAZyme Information: HMPREF1544_07231-t46_1-p1
You are here: Home > Sequence: HMPREF1544_07231-t46_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
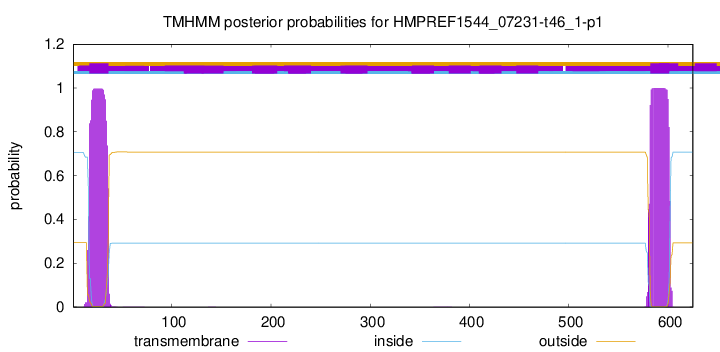
TMHMM annotations
Basic Information help
| Species | Mucor circinelloides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Mucoromycota; Mucoromycetes; ; Mucoraceae; Mucor; Mucor circinelloides | |||||||||||
| CAZyme ID | HMPREF1544_07231-t46_1-p1 | |||||||||||
| CAZy Family | GT1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT34 | 279 | 452 | 1.3e-20 | 0.8495934959349594 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395764 | Synaptobrevin | 1.12e-29 | 513 | 586 | 1 | 74 | Synaptobrevin. |
| 277222 | R-SNARE_VAMP4 | 1.37e-25 | 514 | 579 | 1 | 66 | SNARE motif of VAMP4. The VAMP-4 (vesicle-associated membrane protein 4) protein belongs to the R-SNARE subgroup of SNAREs and interacts with syntaxin 16 (Qa), Vti1a (Qb) and syntaxin 6 (Qc). This complex plays a role in maintenance of Golgi ribbon structure and normal retrograde trafficking from the early endosome to the trans-Golgi network (TGN). SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins contain coiled-coil helices (called SNARE motifs) which mediate the interactions between SNARE proteins, and a transmembrane domain. The SNARE complex mediates membrane fusion, important for trafficking of newly synthesized proteins, recycling of pre-existing proteins and organelle formation. SNARE proteins are classified into four groups, Qa-, Qb-, Qc- and R-SNAREs, depending on whether the residue in the hydrophilic center layer of the four-helical bundle is a glutamine (Q) or arginine (R). Qa-, as well as Qb- and Qc-SNAREs, are localized to target organelle membranes, while R-SNARE is localized to vesicle membranes. They form unique complexes consisting of one member of each subgroup, that mediate fusion between a specific type of vesicles and their target organelle. Their SNARE motifs form twisted and parallel heterotetrameric helix bundles. |
| 277221 | R-SNARE_VAMP8 | 1.73e-23 | 514 | 579 | 1 | 66 | SNARE motif of VAMP8. The lysosomal VAMP8 (vesicle-associated membrane protein 8, also called endobrevin) protein belongs to the R-SNARE subgroup of SNAREs and interacts with STX17 (Qa) and SNAP29 (Qb/Qc). The complex plays a role in autophagosome-lysosome fusion via regulating the transport from early endosomes to multivesicular bodies. Autophagosome transports cytoplasmic materials including cytoplasmic proteins, glycogen, lipids, organelles, and invading bacteria to the lysosome for degradation. SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins contain coiled-coil helices (called SNARE motifs) which mediate the interactions between SNARE proteins, and a transmembrane domain. The SNARE complex mediates membrane fusion, important for trafficking of newly synthesized proteins, recycling of pre-existing proteins and organelle formation. SNARE proteins are classified into four groups, Qa-, Qb-, Qc- and R-SNAREs, depending on whether the residue in the hydrophilic center layer of the four-helical bundle is a glutamine (Q) or arginine (R). Qa-, as well as Qb- and Qc-SNAREs, are localized to target organelle membranes, while R-SNARE is localized to vesicle membranes. They form unique complexes consisting of one member of each subgroup, that mediate fusion between a specific type of vesicles and their target organelle. Their SNARE motifs form twisted and parallel heterotetrameric helix bundles. |
| 277196 | R-SNARE | 2.96e-23 | 516 | 574 | 2 | 60 | SNARE motif, subgroup R-SNARE. SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins consist of coiled-coil helices (called SNARE motifs) which mediate the interactions between SNARE proteins, and a transmembrane domain. The SNARE complex mediates membrane fusion, important for trafficking of newly synthesized proteins, recycling of pre-existing proteins and organelle formation. SNARE proteins are classified into four groups, Qa-, Qb-, Qc- and R-SNAREs, depending on whether the residue in the hydrophilic center layer of the four-helical bundle is a glutamine (Q) or arginine (R). In contrast to Qa-, Qb- and Qc-SNAREs that are localized to target organelle membranes, R-SNAREs are localized to vesicle membranes. They form unique complexes consisting of one member of each subgroup, that mediate fusion between a specific type of vesicles and their target organelle. Their SNARE motifs form twisted and parallel heterotetrameric helix bundles. Examples for members of the Qa SNAREs are syntaxin 18, syntaxin 5, syntaxin 16, and syntaxin 1. |
| 277227 | R-SNARE_Snc1 | 7.57e-23 | 516 | 574 | 2 | 60 | SNARE motif of Snc1. Saccharomyces cerevisiae SNARE protein Snc1p forms a complex with synaptobrevin homolog Sso1p (Qa) and the SNAP-25 homolog Sec9p (Qb/c) which is involved in exocytosis. Snc1 is a member of the R-SNARE subgroup of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, which consist of coiled-coil helices (called SNARE motifs) that mediate the interactions between SNARE proteins, and a transmembrane domain. The SNARE complexes mediate membrane fusion, important for trafficking of newly synthesized proteins, recycling of pre-existing proteins and organelle formation. SNARE proteins are classified into four groups, Qa-, Qb-, Qc- and R-SNAREs, depending on whether the residue in the hydrophilic center layer of the four-helical bundle is a glutamine (Q) or arginine (R). Qa-, as well as Qb- and Qc-SNAREs, are localized to target organelle membranes, while R-SNARE is localized to vesicle membranes. They form unique complexes consisting of one member of each subgroup, that mediate fusion between a specific type of vesicles and their target organelle. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.02e-115 | 38 | 478 | 107 | 652 | |
| 7.07e-108 | 66 | 478 | 155 | 690 | |
| 1.17e-15 | 251 | 395 | 2 | 146 | |
| 2.24e-15 | 202 | 486 | 132 | 419 | |
| 4.35e-15 | 277 | 452 | 105 | 300 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.46e-15 | 508 | 581 | 1 | 74 | Crystal Structure of the Early Endosomal SNARE Complex [Mus musculus] |
|
| 1.65e-13 | 513 | 579 | 2 | 68 | Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens],7BV6_E Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens],7BV6_I Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens],7BV6_M Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens],7BV6_Q Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens],7BV6_U Crystal structure of the autophagic STX17/SNAP29/VAMP8 SNARE complex [Homo sapiens] |
|
| 8.97e-13 | 516 | 574 | 3 | 61 | Structure of the yeast plasma membrane SNARE complex [Saccharomyces cerevisiae],3B5N_E Structure of the yeast plasma membrane SNARE complex [Saccharomyces cerevisiae],3B5N_I Structure of the yeast plasma membrane SNARE complex [Saccharomyces cerevisiae] |
|
| 2.21e-11 | 516 | 578 | 2 | 64 | Crystal structure of autophagic SNARE complex [Homo sapiens] |
|
| 5.17e-11 | 514 | 579 | 29 | 94 | Neuronal Synaptic Fusion Complex [Rattus norvegicus],1SFC_E Neuronal Synaptic Fusion Complex [Rattus norvegicus],1SFC_I Neuronal Synaptic Fusion Complex [Rattus norvegicus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 9.99e-28 | 492 | 591 | 2 | 106 | Synaptobrevin homolog 1 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=syb1 PE=3 SV=1 |
|
| 1.06e-21 | 492 | 588 | 4 | 99 | Synaptobrevin homolog 2 OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=SNC2 PE=1 SV=2 |
|
| 4.38e-17 | 492 | 585 | 4 | 97 | Synaptobrevin homolog 1 OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=SNC1 PE=1 SV=1 |
|
| 8.66e-15 | 511 | 590 | 47 | 126 | Vesicle-associated membrane protein 4 OS=Homo sapiens OX=9606 GN=VAMP4 PE=1 SV=2 |
|
| 1.01e-14 | 507 | 583 | 2 | 78 | Vesicle-associated membrane protein 8 OS=Rattus norvegicus OX=10116 GN=Vamp8 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
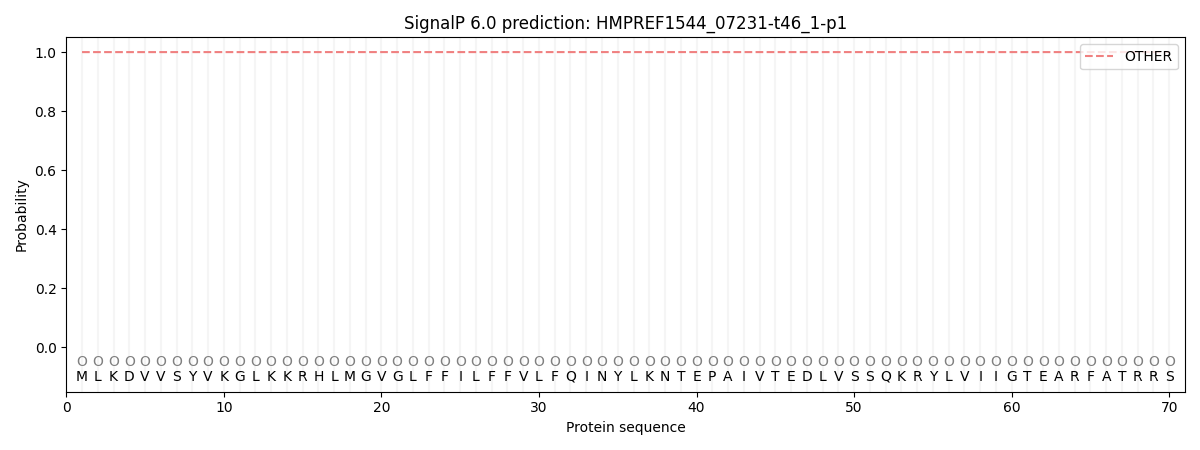
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000027 | 0.000002 |