You are browsing environment: FUNGIDB
CAZyme Information: HMPREF1541_09698-t46_1-p1
You are here: Home > Sequence: HMPREF1541_09698-t46_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Cyphellophora europaea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Cyphellophoraceae; Cyphellophora; Cyphellophora europaea | |||||||||||
| CAZyme ID | HMPREF1541_09698-t46_1-p1 | |||||||||||
| CAZy Family | GT2|GT2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH36 | 76 | 619 | 2.2e-93 | 0.7732558139534884 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 269892 | GH36 | 1.33e-83 | 316 | 610 | 2 | 299 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| 307952 | Melibiase | 4.44e-32 | 302 | 618 | 27 | 347 | Melibiase. Glycoside hydrolase families GH27, GH31 and GH36 form the glycoside hydrolase clan GH-D. Glycoside hydrolase family 36 can be split into 11 families, GH36A to GH36K. This family includes enzymes from GH36A-B and GH36D-K and from GH27. |
| 225882 | GalA | 8.65e-26 | 82 | 619 | 85 | 593 | Alpha-galactosidase [Carbohydrate transport and metabolism]. |
| 269893 | GH27 | 1.37e-10 | 320 | 394 | 5 | 80 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| 407116 | Glyco_hydro_36N | 7.90e-06 | 78 | 181 | 71 | 181 | Glycosyl hydrolase family 36 N-terminal domain. This domain is found at the N-terminus of many family 36 glycoside hydrolases. It has a beta-supersandwich fold. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 7.87e-305 | 1 | 710 | 1 | 714 | |
| 3.07e-304 | 1 | 710 | 1 | 714 | |
| 8.22e-304 | 1 | 710 | 58 | 771 | |
| 2.34e-303 | 1 | 710 | 58 | 771 | |
| 2.34e-303 | 1 | 710 | 58 | 771 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.73e-31 | 81 | 618 | 120 | 639 | Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM, PtCl4 derivative [Lactobacillus acidophilus NCFM],2XN0_B Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM, PtCl4 derivative [Lactobacillus acidophilus NCFM],2XN1_A Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_B Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_C Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_D Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM] |
|
| 7.62e-31 | 81 | 618 | 120 | 639 | Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with galactose [Lactobacillus acidophilus NCFM] |
|
| 6.31e-26 | 92 | 618 | 142 | 648 | SpAga wild type apo structure [Streptococcus pneumoniae TIGR4],6PHV_A Chain A, Alpha-galactosidase [Streptococcus pneumoniae TIGR4] |
|
| 2.57e-25 | 92 | 618 | 142 | 648 | Chain A, Alpha-galactosidase [Streptococcus pneumoniae TIGR4],6PHX_A SpAga D472N structure in complex with raffinose [Streptococcus pneumoniae TIGR4],6PHY_A Chain A, Alpha-galactosidase [Streptococcus pneumoniae TIGR4],6PI0_A AgaD472N-Linear Blood group B type 2 trisaccharide complex structure [Streptococcus pneumoniae TIGR4] |
|
| 5.13e-23 | 91 | 651 | 127 | 667 | Crystal structure of GH36 alpha-galactosidase AgaB from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.95e-30 | 81 | 618 | 120 | 639 | Alpha-galactosidase Mel36A OS=Lactobacillus acidophilus (strain ATCC 700396 / NCK56 / N2 / NCFM) OX=272621 GN=melA PE=1 SV=1 |
|
| 4.90e-23 | 93 | 583 | 128 | 584 | Probable alpha-galactosidase G OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=aglG PE=2 SV=1 |
|
| 2.64e-22 | 91 | 651 | 127 | 667 | Alpha-galactosidase AgaA OS=Geobacillus stearothermophilus OX=1422 GN=agaA PE=1 SV=1 |
|
| 4.65e-22 | 91 | 618 | 129 | 637 | Alpha-galactosidase 1 OS=Pediococcus pentosaceus OX=1255 GN=agaR PE=3 SV=1 |
|
| 2.28e-20 | 90 | 636 | 122 | 650 | Probable alpha-galactosidase G OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=aglG PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
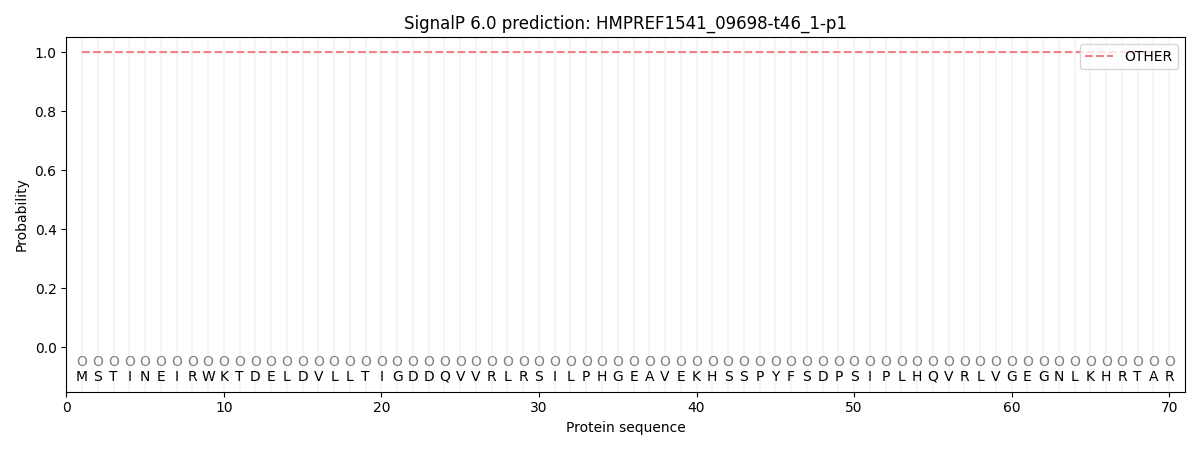
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000061 | 0.000000 |
