You are browsing environment: FUNGIDB
CAZyme Information: HMPREF1541_03088-t46_1-p1
You are here: Home > Sequence: HMPREF1541_03088-t46_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
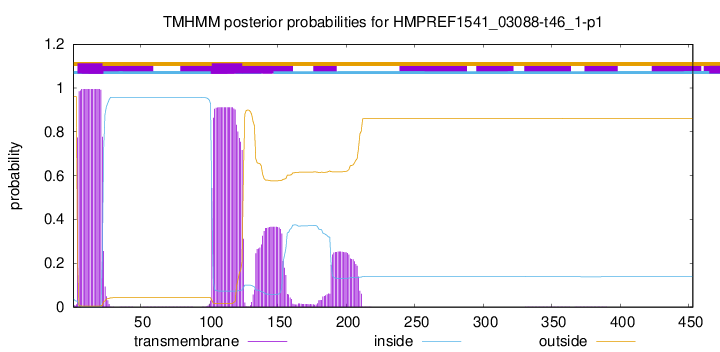
TMHMM annotations
Basic Information help
| Species | Cyphellophora europaea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Cyphellophoraceae; Cyphellophora; Cyphellophora europaea | |||||||||||
| CAZyme ID | HMPREF1541_03088-t46_1-p1 | |||||||||||
| CAZy Family | GH1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 2.4.1.142:10 | 2.4.1.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT33 | 34 | 445 | 2.4e-147 | 0.9929411764705882 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 340843 | GT33_ALG1-like | 0.0 | 35 | 447 | 5 | 411 | chitobiosyldiphosphodolichol beta-mannosyltransferase and similar proteins. This family is most closely related to the GT33 family of glycosyltransferases. The yeast gene ALG1 has been shown to function as a mannosyltransferase that catalyzes the formation of dolichol pyrophosphate (Dol-PP)-GlcNAc2Man from GDP-Man and Dol-PP-Glc-NAc2, and participates in the formation of the lipid-linked precursor oligosaccharide for N-glycosylation. In humans ALG1 has been associated with the congenital disorders of glycosylation (CDG) designated as subtype CDG-Ik. |
| 215155 | PLN02275 | 1.45e-127 | 36 | 413 | 7 | 371 | transferase, transferring glycosyl groups |
| 340831 | GT4_PimA-like | 1.40e-10 | 109 | 449 | 60 | 364 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| 340825 | GT4_WbuB-like | 2.67e-09 | 83 | 438 | 58 | 384 | Escherichia coli WbuB and similar proteins. This family is most closely related to the GT1 family of glycosyltransferases. WbuB in E. coli is involved in the biosynthesis of the O26 O-antigen. It has been proposed to function as an N-acetyl-L-fucosamine (L-FucNAc) transferase. |
| 340816 | Glycosyltransferase_GTB-type | 5.95e-07 | 146 | 399 | 1 | 235 | glycosyltransferase family 1 and related proteins with GTB topology. Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. The structures of the formed glycoconjugates are extremely diverse, reflecting a wide range of biological functions. The members of this family share a common GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.00e-171 | 6 | 447 | 6 | 458 | |
| 7.20e-169 | 7 | 446 | 7 | 455 | |
| 6.50e-167 | 1 | 446 | 1 | 455 | |
| 2.63e-164 | 7 | 447 | 7 | 458 | |
| 4.93e-164 | 1 | 447 | 1 | 456 |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.99e-133 | 71 | 447 | 66 | 443 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_01551 PE=3 SV=1 |
|
| 2.61e-97 | 38 | 451 | 44 | 442 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Yarrowia lipolytica (strain CLIB 122 / E 150) OX=284591 GN=ALG1 PE=3 SV=1 |
|
| 7.23e-96 | 31 | 448 | 22 | 421 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=alg1 PE=3 SV=2 |
|
| 2.82e-89 | 33 | 448 | 54 | 468 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Debaryomyces hansenii (strain ATCC 36239 / CBS 767 / BCRC 21394 / JCM 1990 / NBRC 0083 / IGC 2968) OX=284592 GN=ALG1 PE=3 SV=2 |
|
| 1.41e-88 | 33 | 448 | 51 | 453 | Chitobiosyldiphosphodolichol beta-mannosyltransferase OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=ALG1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
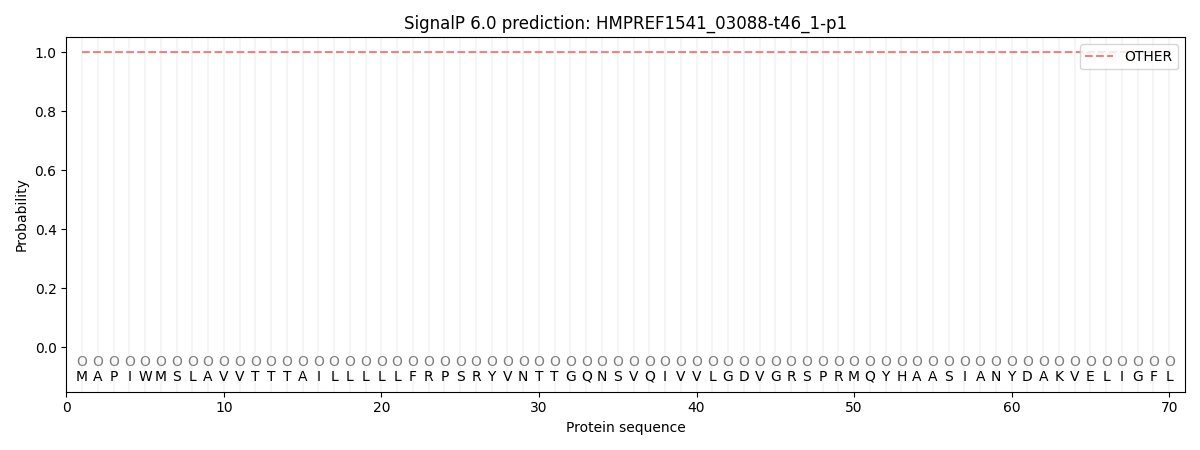
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000043 | 0.000000 |