You are browsing environment: FUNGIDB
CAZyme Information: HCEG_04336-t36_1-p1
You are here: Home > Sequence: HCEG_04336-t36_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
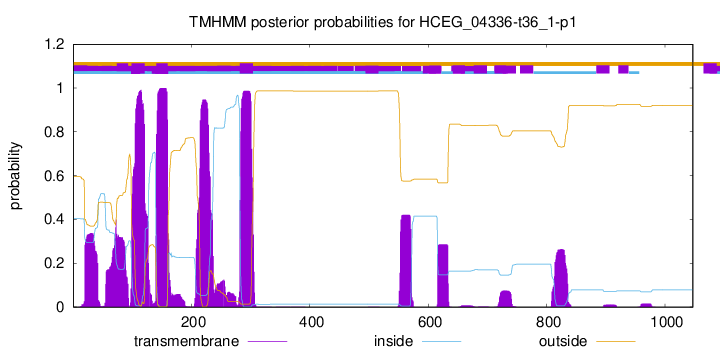
TMHMM annotations
Basic Information help
| Species | Histoplasma capsulatum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Ajellomycetaceae; Histoplasma; Histoplasma capsulatum | |||||||||||
| CAZyme ID | HCEG_04336-t36_1-p1 | |||||||||||
| CAZy Family | GH24 | |||||||||||
| CAZyme Description | GPI mannosyltransferase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT22 | 2 | 364 | 1.1e-58 | 0.8226221079691517 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 410688 | CYP64-like | 1.13e-139 | 617 | 1006 | 1 | 425 | cytochrome P450 family 64-like fungal cytochrome P450s. This group includes Aspergillus flavus cytochrome P450 64 (CYP64), also called O-methylsterigmatocystin (OMST) oxidoreductase or aflatoxin B synthase or aflatoxin biosynthesis protein Q, and similar fungal cytochrome P450s. CYP64 converts OMST to aflatoxin B1 and converts dihydro-O-methylsterigmatocystin (DHOMST) to aflatoxin B2 in the aflatoxin biosynthesis pathway. The CYP64-like subfamily belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 395020 | p450 | 1.72e-46 | 584 | 1008 | 1 | 459 | Cytochrome P450. Cytochrome P450s are haem-thiolate proteins involved in the oxidative degradation of various compounds. They are particularly well known for their role in the degradation of environmental toxins and mutagens. They can be divided into 4 classes, according to the method by which electrons from NAD(P)H are delivered to the catalytic site. Sequence conservation is relatively low within the family - there are only 3 absolutely conserved residues - but their general topography and structural fold are highly conserved. The conserved core is composed of a coil termed the 'meander', a four-helix bundle, helices J and K, and two sets of beta-sheets. These constitute the haem-binding loop (with an absolutely conserved cysteine that serves as the 5th ligand for the haem iron), the proton-transfer groove and the absolutely conserved EXXR motif in helix K. While prokaryotic P450s are soluble proteins, most eukaryotic P450s are associated with microsomal membranes. their general enzymatic function is to catalyze regiospecific and stereospecific oxidation of non-activated hydrocarbons at physiological temperatures. |
| 410710 | CYP1_2-like | 1.23e-42 | 607 | 973 | 56 | 389 | cytochrome P450 families 1 and 2, and similar cytochrome P450s. This model includes cytochrome P450 families 1 (CYP1) and 2 (CYP2), CYP17A1, and CYP21 in vertebrates, as well as insect and crustacean CYPs similar to CYP15A1 and CYP306A1. CYP1 and CYP2 enzymes are involved in the metabolism of endogenous and exogenous compounds such as hormones, xenobiotics, and drugs. CYP17A1 catalyzes the conversion of pregnenolone and progesterone to their 17-alpha-hydroxylated products, while CYP21 catalyzes the 21-hydroxylation of steroids such as progesterone and 17-alpha-hydroxyprogesterone (17-alpha-OH-progesterone) to form 11-deoxycorticosterone and 11-deoxycortisol, respectively. Members of this group belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410653 | CYP17A1-like | 6.19e-42 | 617 | 1006 | 1 | 426 | cytochrome P450 family 17, subfamily A, polypeptide 1, and similar cytochrome P450s. This subfamily contains cytochrome P450 17A1 (CYP17A1 or Cyp17a1), cytochrome P450 21 (CYP21 or Cyp21) and similar proteins. CYP17A1, also called cytochrome P450c17, steroid 17-alpha-hydroxylase (EC 1.14.14.19)/17,20 lyase (EC 1.14.14.32), or 17-alpha-hydroxyprogesterone aldolase, catalyzes the conversion of pregnenolone and progesterone to their 17-alpha-hydroxylated products and subsequently to dehydroepiandrosterone (DHEA) and androstenedione; it catalyzes both the 17-alpha-hydroxylation and the 17,20-lyase reaction. This subfamily also contains CYP21, also called steroid 21-hydroxylase (EC 1.14.14.16) or cytochrome P-450c21 or CYP21A2, catalyzes the 21-hydroxylation of steroids and is required for the adrenal synthesis of mineralocorticoids and glucocorticoids. The CYP17A1-like subfamily belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 281842 | Glyco_transf_22 | 9.38e-42 | 1 | 365 | 65 | 412 | Alg9-like mannosyltransferase family. Members of this family are mannosyltransferase enzymes. At least some members are localized in endoplasmic reticulum and involved in GPI anchor biosynthesis. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 549 | 70 | 618 | |
| 0.0 | 1 | 549 | 70 | 618 | |
| 2.88e-237 | 716 | 1047 | 1 | 332 | |
| 1.43e-182 | 1 | 548 | 70 | 549 | |
| 1.63e-162 | 1 | 544 | 70 | 529 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.13e-40 | 552 | 996 | 3 | 473 | Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida],5E58_B Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida],5E58_C Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida],5E58_D Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida],5E58_E Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida],5E58_F Crystal Structure Of Cytochrome P450 2B35 from Desert Woodrat Neotoma Lepida in complex with 4-(4-chlorophenyl)imidazole [Neotoma lepida] |
|
| 3.00e-37 | 580 | 1017 | 8 | 476 | Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus] |
|
| 4.04e-37 | 580 | 1017 | 8 | 476 | Cytochrome P450 2B4 mutant L437A in complex with 4-(4-chlorophenyl)imidazole [Oryctolagus cuniculus],3TK3_B Cytochrome P450 2B4 mutant L437A in complex with 4-(4-chlorophenyl)imidazole [Oryctolagus cuniculus],3TK3_C Cytochrome P450 2B4 mutant L437A in complex with 4-(4-chlorophenyl)imidazole [Oryctolagus cuniculus],3TK3_D Cytochrome P450 2B4 mutant L437A in complex with 4-(4-chlorophenyl)imidazole [Oryctolagus cuniculus] |
|
| 7.33e-37 | 580 | 1017 | 8 | 476 | Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],2BDM_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G5N_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G5N_B Chain B, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G5N_C Chain C, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G5N_D Chain D, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G93_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G93_B Chain B, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G93_C Chain C, Cytochrome P450 2B4 [Oryctolagus cuniculus],3G93_D Chain D, Cytochrome P450 2B4 [Oryctolagus cuniculus],3KW4_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3ME6_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3ME6_B Chain B, Cytochrome P450 2B4 [Oryctolagus cuniculus],3ME6_C Chain C, Cytochrome P450 2B4 [Oryctolagus cuniculus],3ME6_D Chain D, Cytochrome P450 2B4 [Oryctolagus cuniculus],3MVR_A Chain A, Cytochrome P450 2B4 [Oryctolagus cuniculus],3MVR_B Chain B, Cytochrome P450 2B4 [Oryctolagus cuniculus],3R1A_A Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_B Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_C Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_D Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_E Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_F Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_G Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1A_H Closed crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1B_A Open crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1B_B Open crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1B_C Open crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3R1B_D Open crystal structure of cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene [Oryctolagus cuniculus],3TMZ_A Crystal Structure of P450 2B4(H226Y) in complex with Amlodipine [Oryctolagus cuniculus],3UAS_A Cytochrome P450 2B4 covalently bound to the mechanism-based inactivator 9-ethynylphenanthrene [Oryctolagus cuniculus],4JLT_A Crystal structure of P450 2B4(H226Y) in complex with paroxetine [Oryctolagus cuniculus] |
|
| 1.27e-36 | 554 | 1009 | 3 | 487 | Structure of CYP2B4 F244W in a ligand free conformation [Oryctolagus cuniculus],5EM4_B Structure of CYP2B4 F244W in a ligand free conformation [Oryctolagus cuniculus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.19e-160 | 1 | 544 | 70 | 535 | GPI mannosyltransferase 4 OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=smp3 PE=3 SV=1 |
|
| 7.79e-153 | 565 | 1039 | 8 | 523 | Cytochrome P450 monooxygenase CLM2 OS=Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) OX=229533 GN=CLM2 PE=3 SV=1 |
|
| 2.92e-147 | 1 | 544 | 70 | 534 | GPI mannosyltransferase 4 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=smp3 PE=3 SV=2 |
|
| 5.96e-147 | 1 | 544 | 70 | 539 | GPI mannosyltransferase 4 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=smp3 PE=3 SV=2 |
|
| 4.52e-146 | 564 | 1038 | 17 | 531 | Multifunctional cytochrome P450 monooxygenase af510 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=af510 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
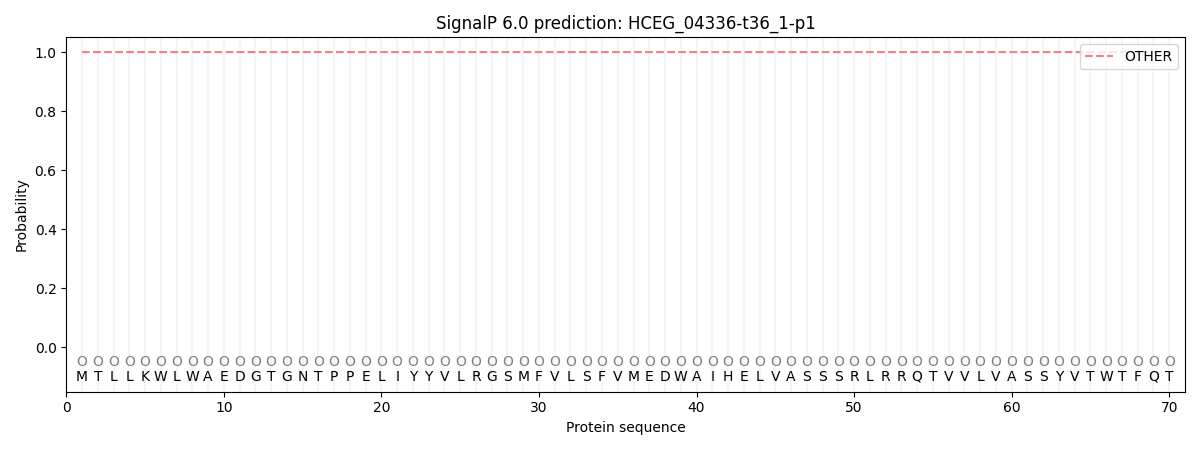
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000021 | 0.000001 |