You are browsing environment: FUNGIDB
CAZyme Information: H310_02386-t26_1-p1
You are here: Home > Sequence: H310_02386-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aphanomyces invadans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Saprolegniaceae; Aphanomyces; Aphanomyces invadans | |||||||||||
| CAZyme ID | H310_02386-t26_1-p1 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT41 | 140 | 792 | 4.6e-83 | 0.6425531914893617 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 226428 | Spy | 3.58e-31 | 203 | 775 | 26 | 591 | Predicted O-linked N-acetylglucosamine transferase, SPINDLY family [Posttranslational modification, protein turnover, chaperones]. |
| 276809 | TPR | 1.12e-14 | 202 | 295 | 4 | 97 | Tetratricopeptide repeat. The Tetratricopeptide repeat (TPR) typically contains 34 amino acids and is found in a variety of organisms including bacteria, cyanobacteria, yeast, fungi, plants, and humans. It is present in a variety of proteins including those involved in chaperone, cell-cycle, transcription, and protein transport complexes. The number of TPR motifs varies among proteins. Those containing 5-6 tandem repeats generate a right-handed helical structure with an amphipathic channel that is thought to accommodate an alpha-helix of a target protein. It has been proposed that TPR proteins preferentially interact with WD-40 repeat proteins, but in many instances several TPR-proteins seem to aggregate to multi-protein complexes. |
| 274350 | PEP_TPR_lipo | 4.32e-14 | 53 | 327 | 288 | 593 | putative PEP-CTERM system TPR-repeat lipoprotein. This protein family occurs in strictly within a subset of Gram-negative bacterial species with the proposed PEP-CTERM/exosortase system, analogous to the LPXTG/sortase system common in Gram-positive bacteria. This protein occurs in a species if and only if a transmembrane histidine kinase (TIGR02916) and a DNA-binding response regulator (TIGR02915) also occur. The present of tetratricopeptide repeats (TPR) suggests protein-protein interaction, possibly for the regulation of PEP-CTERM protein expression, since many PEP-CTERM proteins in these genomes are preceded by a proposed DNA binding site for the response regulator. |
| 276809 | TPR | 7.97e-14 | 235 | 328 | 3 | 96 | Tetratricopeptide repeat. The Tetratricopeptide repeat (TPR) typically contains 34 amino acids and is found in a variety of organisms including bacteria, cyanobacteria, yeast, fungi, plants, and humans. It is present in a variety of proteins including those involved in chaperone, cell-cycle, transcription, and protein transport complexes. The number of TPR motifs varies among proteins. Those containing 5-6 tandem repeats generate a right-handed helical structure with an amphipathic channel that is thought to accommodate an alpha-helix of a target protein. It has been proposed that TPR proteins preferentially interact with WD-40 repeat proteins, but in many instances several TPR-proteins seem to aggregate to multi-protein complexes. |
| 223533 | TPR | 1.26e-13 | 74 | 298 | 37 | 267 | Tetratricopeptide (TPR) repeat [General function prediction only]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 5.75e-89 | 338 | 799 | 139 | 588 | |
| 6.05e-78 | 315 | 797 | 419 | 885 | |
| 6.05e-78 | 315 | 797 | 419 | 885 | |
| 1.87e-75 | 314 | 798 | 182 | 652 | |
| 9.15e-74 | 311 | 799 | 456 | 925 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.65e-14 | 354 | 761 | 72 | 478 | Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_B Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_C Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_D Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum] |
|
| 1.22e-08 | 601 | 791 | 508 | 694 | The human O-GlcNAc transferase in complex with a thiol-linked bisubstrate inhibitor [Homo sapiens] |
|
| 1.22e-08 | 601 | 791 | 514 | 700 | Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_B Chain B, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE3_D Chain D, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],3PE4_A Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3PE4_C Structure of human O-GlcNAc transferase and its complex with a peptide substrate [Homo sapiens],3TAX_A A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],3TAX_C A Neutral Diphosphate Mimic Crosslinks the Active Site of Human O-GlcNAc Transferase [Homo sapiens],4AY5_A Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_B Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_C Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY5_D Human O-GlcNAc transferase (OGT) in complex with UDP and glycopeptide [Homo sapiens],4AY6_A Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_B Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_C Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4AY6_D Human O-GlcNAc transferase (OGT) in complex with UDP-5SGlcNAc and substrate peptide [Homo sapiens],4CDR_A Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_B Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_C Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4CDR_D Human O-GlcNAc transferase in complex with a bisubstrate inhibitor, Goblin1 [Homo sapiens],4GYW_A Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYW_C Crystal structure of human O-GlcNAc Transferase in complex with UDP and a glycopeptide [Homo sapiens],4GYY_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GYY_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc and a peptide substrate [Homo sapiens],4GZ3_A Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ3_C Crystal structure of human O-GlcNAc Transferase with UDP and a thioglycopeptide [Homo sapiens],4GZ5_A Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_B Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_C Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ5_D Crystal structure of human O-GlcNAc Transferase with UDP-GlcNAc [Homo sapiens],4GZ6_A Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_B Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_C Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4GZ6_D Crystal structure of human O-GlcNAc Transferase with UDP-5SGlcNAc [Homo sapiens],4N39_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) [Homo sapiens],4N3A_A Crystal Structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (1-26)E10A [Homo sapiens],4N3B_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26)E10Q and UDP-5SGlcNAc [Homo sapiens],4N3C_A Crystal Structure of human O-GlcNAc Transferase bound to a peptide from HCF-1 pro-repeat2(1-26) and UDP-GlcNAc [Homo sapiens],4XI9_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XI9_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RBL2) [Homo sapiens],4XIF_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_B Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_C Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],4XIF_D Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (keratin-7) [Homo sapiens],5BNW_A The active site of O-GlcNAc transferase imposes constraints on substrate sequence [Homo sapiens],5C1D_A Human OGT in complex with UDP-5S-GlcNAc and substrate peptide (RB2L) [Homo sapiens],5VIE_A Chain A, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIE_C Chain C, UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit [Homo sapiens],5VIF_A Electrophilic probes for deciphering substrate recognition by O-GlcNAc transferase [Homo sapiens],6E37_A O-GlcNAc Transferase in complex with covalent inhibitor [Homo sapiens],6MA1_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 4a [Homo sapiens],6MA2_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor ent-1a [Homo sapiens],6MA3_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 2a [Homo sapiens],6MA4_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 3a [Homo sapiens],6MA5_A Crystal structure of human O-GlcNAc transferase bound to a peptide from HCF-1 pro-repeat 2 (11-26) and inhibitor 1a [Homo sapiens] |
|
| 1.22e-08 | 601 | 791 | 509 | 695 | The human O-GlcNAc transferase in complex with a bisubstrate inhibitor [Homo sapiens] |
|
| 1.22e-08 | 601 | 791 | 514 | 700 | Catalytic deficiency of O-GlcNAc transferase leads to X-linked intellectual disability [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.05e-14 | 534 | 774 | 588 | 821 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Solanum lycopersicum OX=4081 GN=SPY PE=2 SV=1 |
|
| 3.54e-14 | 534 | 774 | 588 | 821 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Petunia hybrida OX=4102 GN=SPY PE=2 SV=1 |
|
| 3.57e-14 | 534 | 774 | 574 | 807 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Hordeum vulgare OX=4513 GN=SPY PE=2 SV=1 |
|
| 1.62e-12 | 534 | 761 | 583 | 803 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Arabidopsis thaliana OX=3702 GN=SPY PE=1 SV=1 |
|
| 3.72e-12 | 534 | 768 | 574 | 801 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Oryza sativa subsp. japonica OX=39947 GN=SPY PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
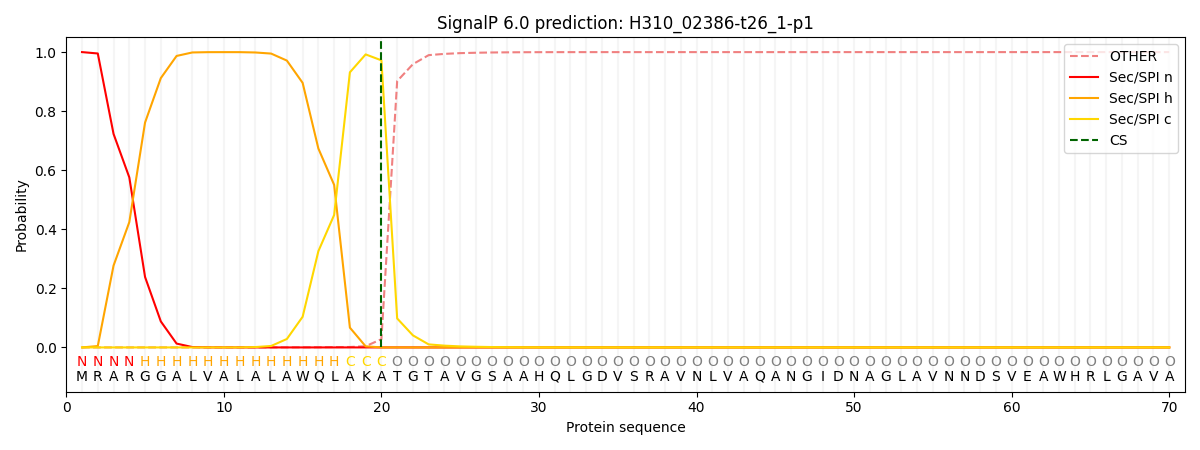
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000561 | 0.999420 | CS pos: 20-21. Pr: 0.9721 |
