You are browsing environment: FUNGIDB
CAZyme Information: FUN_010688-T1-p1
You are here: Home > Sequence: FUN_010688-T1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
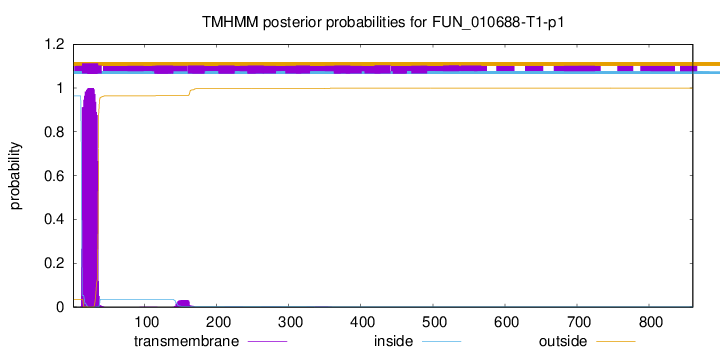
TMHMM annotations
Basic Information help
| Species | Rhizophagus irregularis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Mucoromycota; Glomeromycetes; ; Glomeraceae; Rhizophagus; Rhizophagus irregularis | |||||||||||
| CAZyme ID | FUN_010688-T1-p1 | |||||||||||
| CAZy Family | GH5 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH47 | 57 | 491 | 2.7e-145 | 0.9932735426008968 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396217 | Glyco_hydro_47 | 8.65e-148 | 57 | 489 | 1 | 449 | Glycosyl hydrolase family 47. Members of this family are alpha-mannosidases that catalyze the hydrolysis of the terminal 1,2-linked alpha-D-mannose residues in the oligo-mannose oligosaccharide Man(9)(GlcNAc)(2). |
| 240427 | PTZ00470 | 7.30e-77 | 38 | 496 | 60 | 521 | glycoside hydrolase family 47 protein; Provisional |
| 238300 | PA | 5.46e-15 | 717 | 807 | 35 | 121 | PA: Protease-associated (PA) domain. The PA domain is an insert domain in a diverse fraction of proteases. The significance of the PA domain to many of the proteins in which it is inserted is undetermined. It may be a protein-protein interaction domain. At peptidase active sites, the PA domain may participate in substrate binding and/or promoting conformational changes, which influence the stability and accessibility of the site to substrate. Proteins into which the PA domain is inserted include the following: i) various signal peptide peptidases including, hSPPL2a and 2b which catalyze the intramembrane proteolysis of tumor necrosis factor alpha, ii) various proteins containing a C3H2C3 RING finger including, Arabidopsis ReMembR-H2 protein and various E3 ubiquitin ligases such as human GRAIL (gene related to anergy in lymphocytes), iii) EDEM3 (ER-degradation-enhancing mannosidase-like 3 protein), iv) various plant vacuolar sorting receptors such as Pisum sativum BP-80, v) glutamate carboxypeptidase II (GCPII), vi) yeast aminopeptidase Y, vii) Vibrio metschnikovii VapT, a sodium dodecyl sulfate (SDS) resistant extracellular alkaline serine protease, viii) lactocepin (a cell envelope-associated protease from Lactobacillus paracasei subsp. paracasei NCDO 151), ix) various subtilisin-like proteases such as melon Cucumisin, and x) human TfR (transferrin receptor) 1 and 2. |
| 239041 | PA_EDEM3_like | 2.40e-13 | 691 | 807 | 2 | 121 | PA_EDEM3_like: protease associated domain (PA) domain-containing EDEM3-like proteins. This group contains various PA domain-containing proteins similar to mouse EDEM3 (ER-degradation-enhancing mannosidase-like 3 protein). EDEM3 contains a region, similar to Class I alpha-mannosidases (gylcosyl hydrolase family 47), N-terminal to the PA domain. EDEM3 accelerates glycoprotein ERAD (ER-associated degradation). In transfected mammalian cells, overexpression of EDEM3 enhances the mannose trimming from the N-glycans, of a model misfolded protein [alpha1-antitrypsin null (Hong Kong)] as well as, from total glycoproteins. Mannose trimming appears to be involved in the selection of ERAD substrates. EDEM3 has a different specificity of trimming than ER alpha-mannosidase 1. The significance of the PA domain to EDEM3 has not been ascertained. It may be a protein-protein interaction domain. At peptidase active sites, the PA domain may participate in substrate binding and/or promoting conformational changes, which influence the stability and accessibility of the site to substrate. |
| 240122 | PA_subtilisin_1 | 8.61e-13 | 694 | 806 | 3 | 112 | PA_subtilisin_1: Protease-associated domain containing subtilisin-like proteases, subgroup 1. A subgroup of PA domain-containing subtilisin-like proteases. The significance of the PA domain to many of the proteins in which it is inserted is undetermined. It may be a protein-protein interaction domain. At peptidase active sites, the PA domain may participate in substrate binding and/or promoting conformational changes, which influence the stability and accessibility of the site to substrate. Proteins into which the PA domain is inserted include the following subtilisin-like proteases: i) melon cucumisin, ii) Arabidopsis thaliana Ara12, iii) Alnus glutinosa ag12, iv) members of the tomato P69 family, and v) tomato LeSBT2. However, these proteins belong to other subtilisin-like subgroups. Relatively little is known about proteins in this subgroup. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 5.45e-228 | 57 | 631 | 1 | 574 | |
| 4.25e-196 | 43 | 803 | 45 | 819 | |
| 3.33e-192 | 43 | 803 | 45 | 819 | |
| 6.24e-188 | 40 | 803 | 41 | 862 | |
| 5.19e-180 | 45 | 776 | 30 | 785 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.15e-51 | 51 | 489 | 23 | 463 | Structure of mouse Golgi alpha-1,2-mannosidase IA and Man9GlcNAc2-PA complex [Mus musculus],5KKB_B Structure of mouse Golgi alpha-1,2-mannosidase IA and Man9GlcNAc2-PA complex [Mus musculus] |
|
| 2.60e-51 | 51 | 489 | 21 | 461 | Structure of mouse Golgi alpha-1,2-mannosidase IA reveals the molecular basis for substrate specificity among Class I enzymes (family 47 glycosidases) [Mus musculus] |
|
| 5.53e-49 | 56 | 489 | 11 | 447 | Crystal structure of the class I human endoplasmic reticulum 1,2-alpha-mannosidase and Man9GlcNAc2-PA complex [Homo sapiens] |
|
| 6.28e-49 | 56 | 489 | 16 | 452 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase [Homo sapiens] |
|
| 6.55e-49 | 56 | 489 | 16 | 452 | Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With 1-Deoxymannojirimycin [Homo sapiens],1FO3_A Crystal Structure Of Human Class I Alpha1,2-Mannosidase In Complex With Kifunensine [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.12e-170 | 50 | 491 | 130 | 584 | ER degradation-enhancing alpha-mannosidase-like protein 1 OS=Homo sapiens OX=9606 GN=EDEM1 PE=1 SV=1 |
|
| 1.34e-170 | 50 | 496 | 125 | 584 | ER degradation-enhancing alpha-mannosidase-like protein 1 OS=Mus musculus OX=10090 GN=Edem1 PE=1 SV=1 |
|
| 2.13e-166 | 39 | 567 | 24 | 548 | ER degradation-enhancing alpha-mannosidase-like protein 1 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=mnl1 PE=3 SV=2 |
|
| 1.65e-137 | 27 | 832 | 15 | 797 | ER degradation-enhancing alpha-mannosidase-like protein 3 OS=Xenopus laevis OX=8355 GN=edem3 PE=2 SV=2 |
|
| 5.38e-136 | 42 | 807 | 45 | 785 | ER degradation-enhancing alpha-mannosidase-like protein 3 OS=Mus musculus OX=10090 GN=Edem3 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
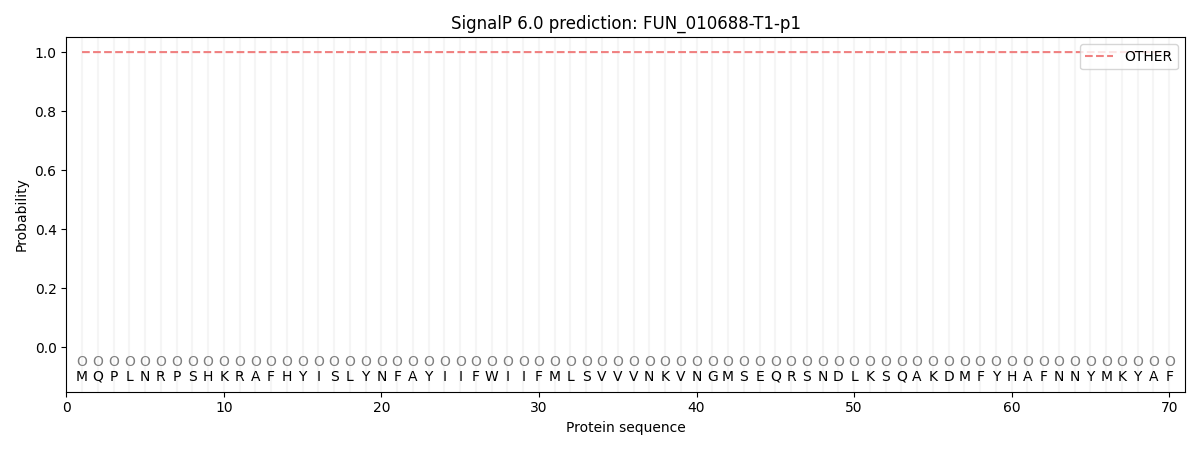
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000020 | 0.000002 |